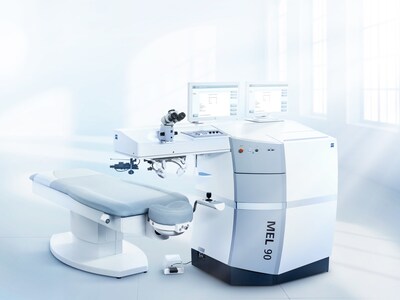
ZEISS MEL 90 excimer laser receives U.S. FDA approval; completes Corneal Refractive Workflow
ZEISS Medical Technology announced FDA approval for its MEL 90 excimer laser, receiving simultaneous approval for treating myopia, hyperopia, and mixed astigmatism. The technology integrates with the VISUMAX 800 with SMILE pro, enhancing ZEISS' laser vision correction market leadership.
The MEL 90 features Triple-A (Advanced Ablation Algorithm) technology, offering high accuracy and tissue-saving ablation. It can ablate 1 diopter in 1.3 seconds during LASIK procedures at 500 Hz. The system includes an active eye tracker, intuitive interface, and flexible touch screen positioning for improved workflow efficiency.
This approval completes ZEISS' Corneal Refractive Workflow, providing U.S. surgeons with an integrated system for enhanced patient outcomes and practice efficiency. The technology is immediately available in the U.S. market.
ZEISS Medical Technology ha annunciato l'approvazione da parte della FDA per il suo laser a eccimeri MEL 90, ottenendo contemporaneamente l'approvazione per il trattamento della miopia, dell'ipermetropia e dell'astigmatismo misto. La tecnologia si integra con il VISUMAX 800 con SMILE pro, rafforzando la leadership di ZEISS nel mercato della correzione visiva laser.
Il MEL 90 presenta la tecnologia Triple-A (Advanced Ablation Algorithm), che offre un'alta precisione e ablazione a risparmio di tessuto. Può ablare 1 diottria in 1,3 secondi durante le procedure LASIK a 500 Hz. Il sistema include un tracciante oculare attivo, un'interfaccia intuitiva e una posizione dello schermo touch flessibile per migliorare l'efficienza del flusso di lavoro.
Questa approvazione completa il Workflow Refrattivo Corneale di ZEISS, fornendo ai chirurghi statunitensi un sistema integrato per migliorare i risultati per i pazienti e l'efficienza della pratica. La tecnologia è immediatamente disponibile nel mercato statunitense.
ZEISS Medical Technology anunció la aprobación de la FDA para su láser excimer MEL 90, recibiendo la aprobación simultánea para tratar la miopía, la hipermetropía y el astigmatismo mixto. La tecnología se integra con el VISUMAX 800 con SMILE pro, mejorando el liderazgo de ZEISS en el mercado de corrección visual con láser.
El MEL 90 cuenta con la tecnología Triple-A (Advanced Ablation Algorithm), que ofrece alta precisión y ablación que ahorra tejido. Puede ablacionar 1 dioptría en 1,3 segundos durante procedimientos LASIK a 500 Hz. El sistema incluye un rastreador ocular activo, una interfaz intuitiva y posicionamiento de pantalla táctil flexible para mejorar la eficiencia del flujo de trabajo.
Esta aprobación completa el Flujo de Trabajo Refractivo Corneal de ZEISS, proporcionando a los cirujanos en EE. UU. un sistema integrado para mejorar los resultados del paciente y la eficiencia de la práctica. La tecnología está disponible de inmediato en el mercado estadounidense.
ZEISS Medical Technology는 MEL 90 엑시머 레이저에 대한 FDA 승인을 발표했으며, 근시, 원시 및 혼합 난시 치료를 위한 동시 승인을 받았습니다. 이 기술은 VISUMAX 800과 SMILE pro와 통합되어 ZEISS의 레이저 시력 교정 시장 리더십을 강화합니다.
MEL 90은 고급 절제 알고리즘(Triple-A) 기술을 특징으로 하며, 높은 정밀도와 조직 절약적인 절제를 제공합니다. LASIK 절차에서 500Hz로 1.3초 만에 1디옵터를 절제할 수 있습니다. 이 시스템은 능동적 눈 추적기, 직관적인 인터페이스 및 향상된 작업 흐름 효율성을 위한 유연한 터치 스크린 위치를 포함합니다.
이 승인은 ZEISS의 각막 굴절 흐름을 완성하여 미국의 외과 의사에게 환자 결과와 실무 효율성을 개선하기 위한 통합 시스템을 제공합니다. 이 기술은 즉시 미국 시장에서 사용할 수 있습니다.
ZEISS Medical Technology a annoncé l'approbation de la FDA pour son laser excimer MEL 90, recevant une approbation simultanée pour le traitement de la myopie, de l'hypermétropie et de l'astigmatisme mixte. La technologie s'intègre au VISUMAX 800 avec SMILE pro, renforçant la position de leader de ZEISS sur le marché de la correction de la vue au laser.
Le MEL 90 présente la technologie Triple-A (Advanced Ablation Algorithm), offrant une grande précision et une ablation respectueuse des tissus. Il peut ablater 1 dioptrie en 1,3 seconde lors de procédures LASIK à 500 Hz. Le système comprend un traqueur oculaire actif, une interface intuitive et un positionnement flexible de l'écran tactile pour améliorer l'efficacité des flux de travail.
Cette approbation complète le Workflow Réfractif Corneal de ZEISS, fournissant aux chirurgiens américains un système intégré pour améliorer les résultats pour les patients et l'efficacité de la pratique. La technologie est immédiatement disponible sur le marché américain.
ZEISS Medical Technology hat die FDA-Zulassung für ihren MEL 90 Excimer-Laser bekannt gegeben und gleichzeitig die Genehmigung zur Behandlung von Myopie, Hyperopie und gemischtem Astigmatismus erhalten. Die Technologie integriert sich mit dem VISUMAX 800 mit SMILE pro und stärkt die Marktführerschaft von ZEISS in der lasergestützten Sehkorrektur.
Der MEL 90 verfügt über die Triple-A (Advanced Ablation Algorithm) Technologie, die hohe Genauigkeit und gewebeschonende Ablation bietet. Er kann während LASIK-Verfahren bei 500 Hz in 1,3 Sekunden 1 Dioptrie abtragen. Das System umfasst einen aktiven Augentracker, eine intuitive Benutzeroberfläche und flexible Touchscreen-Positionierung zur Verbesserung der Arbeitsablaufeffizienz.
Diese Genehmigung vervollständigt den Hornhaut-Refraktive Workflow von ZEISS und bietet Chirurgen in den USA ein integriertes System für verbesserte Patientenergebnisse und Praxiseffizienz. Die Technologie ist sofort auf dem US-Markt erhältlich.
- FDA approval received for all three major eye correction indications simultaneously
- Fast ablation speed - 1 diopter in 1.3 seconds during LASIK procedures
- Integration with existing VISUMAX 800 system enhances workflow efficiency
- Advanced Triple-A technology offers high accuracy and tissue-saving features
- None.
The excimer laser complements the ZEISS VISUMAX 800 with ZEISS SMILE pro, extending ZEISS' LVC market leadership with treatment for myopia, hyperopia, and mixed astigmatism.
"The increasing global adoption of laser vision correction reflects the advancements and positive impact the technology continues to have on the quality of life for patients," says Magnus Reibenspiess, Head of Strategic Business Unit Ophthalmology at ZEISS Medical Technology. "With the integration of the ZEISS MEL 90, surgeons can confidently care for their patients with greater workflow efficiency and performance with enhanced outcomes."
"ZEISS continues to break new ground as a leader in the LVC market, reflecting our ongoing commitment to and support of
"The FDA approval of the ZEISS MEL 90 excimer laser is a game changer for refractive surgery in the
Reliable Outcomes: The ZEISS MEL 90 offers Triple-A (Advanced Ablation Algorithm), which is a single ablation profile for a wide range of sphero-cylindrical (SCA) corrections that simplifies treatment planning. Triple-A offers a high degree of accuracy, reproducibility and predictability, as well as advantages such as tissue-saving ablation. Its preinstalled functions also offer surgeons optimal support for achieving excellent treatment results and gentle treatments of standard, higher and lower levels of ametropia and astigmatism.
Fast Ablation Speed: The ZEISS MEL 90 offers a truly customized power package. When performing LASIK for myopia at 500 Hz, the ZEISS MEL 90 can intra-operatively ablate 1 diopter in as little as 1.3 seconds.1 Intuitive system guidance and speedy treatment planning allow for additional time savings. The ZEISS MEL 90 excimer laser technology is equipped with an active eye tracker which delivers an excellent response time, providing a high level of treatment safety with very stable results.
Easy to Use: New capabilities of the ZEISS MEL 90 excimer laser greatly simplify the interactions between the surgeon, the assisting staff and the system technology. The excimer laser can be configured to precisely match the surgeon and the OR team's needs. The system's simple, intuitive graphic user interface supports fast treatment procedures, and the touch screen can be flexibly positioned wherever it is needed to enable the surgeon to have good ergonomic sitting posture throughout an operation. A second, optional touch screen can be configured to optimally complement the treatment routine of the OR team. An HD video port, network printer connection, and PDF export capabilities offer additional workflow support.
With the ZEISS MEL 90, the company expands its offerings for
The ZEISS MEL 90 excimer laser is currently available in the
1LASIK myopia, optical zone 6 mm.
Not all products, services or offers are approved or offered in every market and approved labeling and instructions may vary from one country to another. For country-specific product information, see the appropriate country website. Product specifications are subject to change in design and scope of delivery as a result of ongoing technical development. The statements of the healthcare professionals reflect only their personal opinions and experiences and do not necessarily reflect the opinion of any institution that they are affiliated with. The healthcare professionals alone are responsible for the content of their experience reported and any potential resulting infringements. Carl Zeiss Meditec AG and its affiliates to not have clinical evidence supporting the opinions and statements of the health care professionals nor accept any responsibility or liability of the healthcare professionals' content. The healthcare professionals have a contractual or other financial relationship with Carl Zeiss Meditec AG and its affiliates and have received financial support.
Brief profile
Carl Zeiss Meditec AG (ISIN: DE0005313704), which is listed on the MDAX and TecDAX of the German stock exchange, is one of the world's leading medical technology companies. The Company supplies innovative technologies and application-oriented solutions designed to help doctors improve the quality of life of their patients. The Company offers complete solutions, including implants and consumables, to diagnose and treat eye diseases. The Company creates innovative visualization solutions in the field of microsurgery. With 5,730 employees worldwide, the Group generated revenue of
The Group's head office is located in Jena,
For more information visit our website at www.zeiss.com/med
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/zeiss-mel-90-excimer-laser-receives-us-fda-approval-completes-corneal-refractive-workflow-302349058.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/zeiss-mel-90-excimer-laser-receives-us-fda-approval-completes-corneal-refractive-workflow-302349058.html
SOURCE Carl Zeiss Meditec AG