Chemomab Therapeutics Announces Positive Phase 2 Trial Results: CM-101 Achieves Primary and Secondary Endpoints Demonstrating Anti-Fibrotic, Anti-Inflammatory and Anti-Cholestatic Effects in Patients with Primary Sclerosing Cholangitis
Chemomab Therapeutics (Nasdaq: CMMB) has reported positive topline results from its Phase 2 SPRING trial of CM-101, a first-in-class monoclonal antibody, in patients with primary sclerosing cholangitis (PSC). The trial met its primary endpoint of safety and tolerability and demonstrated anti-fibrotic, anti-inflammatory, and anti-cholestatic effects across multiple secondary efficacy endpoints.
Key findings include:
- Statistically significant improvement in liver stiffness, a important PSC disease marker
- Reduction in total bilirubin, an important marker of cholestasis and liver health
- Improvements in pruritis, a cholestatic indicator relevant to patients
- Favorable safety profile and general tolerability over the 15-week treatment period
The company plans to advance CM-101 to a Phase 3 PSC trial in 2025, following discussions with the FDA later this year.
Chemomab Therapeutics (Nasdaq: CMMB) ha riportato risultati positivi di sintesi dal suo studio di Fase 2 SPRING riguardante CM-101, un anticorpo monoclonale di prima classe, in pazienti con colangite sclerosante primaria (PSC). Lo studio ha raggiunto il punto finale principale di sicurezza e tollerabilità e ha dimostrato effetti anti-fibrotici, anti-infiammatori e anti-colestatici in vari endpoint secondari di efficacia.
I risultati chiave includono:
- Un miglioramento statisticamente significativo nella rigidità epatica, un importante marcatore della malattia PSC
- Una riduzione del bilirubina totale, un marcatore importante della colestasi e della salute epatica
- Miglioramenti nel prurito, un indicatore colestatico rilevante per i pazienti
- Un profilo di sicurezza favorevole e una generale tollerabilità nel periodo di trattamento di 15 settimane
La società prevede di avanzare CM-101 a un studio di Fase 3 sulla PSC nel 2025, dopo aver discusso con la FDA entro la fine di quest'anno.
Chemomab Therapeutics (Nasdaq: CMMB) ha reportado resultados positivos preliminares de su ensayo de Fase 2 SPRING de CM-101, un anticuerpo monoclonal de primera clase, en pacientes con colangitis esclerosante primaria (PSC). El ensayo cumplió con su objetivo primario de seguridad y tolerabilidad y demostró efectos anti-fibróticos, anti-inflamatorios y anti-colestáticos en múltiples puntos finales secundarios de eficacia.
Los hallazgos clave incluyen:
- Mejora estadísticamente significativa en la rigidez hepática, un importante marcador de la enfermedad PSC
- Reducción en la bilirrubina total, un marcador importante de colestasis y salud hepática
- Mejoras en prurito, un indicador colestático relevante para los pacientes
- Perfil de seguridad favorable y buena tolerabilidad durante el período de tratamiento de 15 semanas
La compañía planea avanzar CM-101 a un ensayo de Fase 3 sobre PSC en 2025, tras conversaciones con la FDA a finales de este año.
Chemomab Therapeutics (Nasdaq: CMMB)는 긍정적인 주요 결과를 보고했습니다. 이는 일차성 경화 담관염 (PSC) 환자에서 사용되는 최초의 클래스인 단클론 항체인 CM-101에 대한 2상 SPRING 시험에서 얻어진 것입니다. 이 시험은 안전성 및 내약성의 주요 목표를 달성했으며, 여러 2차 효능 목표에 걸쳐 항 섬유화, 항 염증 및 항 담즙 정체 효과를 입증했습니다.
주요 발견 사항은 다음과 같습니다:
- 간 경직도, PSC 질병의 주요 지표에서 통계적으로 유의한 개선
- 총 빌리루빈 감소, 담즙 정체 및 간 건강의 중요한 지표
- 환자와 관련된 담즙 정체 지표인 가려움증의 개선
- 15주 치료 기간 동안의 유리한 안전성 프로필 및 일반적인 내약성
회사는 올해 말 FDA와의 논의 후 2025년에 CM-101을 3상 PSC 시험으로 진행할 계획입니다.
Chemomab Therapeutics (Nasdaq: CMMB) a annoncé des résultats positifs préliminaires de son essai de Phase 2 SPRING sur CM-101, un anticorps monoclonal de première classe, chez des patients souffrant de cholangite sclérosante primitive (PSC). L'essai a atteint son objectif principal de sécurité et de tolérance et a montré des effets anti-fibrotiques, anti-inflammatoires et anti-cholestatiques sur plusieurs endpoints secondaires d'efficacité.
Les résultats clés incluent :
- Amélioration statistiquement significative de la rigidité hépatique, un marqueur important de la maladie PSC
- Réduction de la bilirubine totale, un marqueur essentiel de cholestase et de santé hépatique
- Améliorations au niveau du prurit, un indicateur cholestatique pertinent pour les patients
- Profil de sécurité favorable et bonne tolérance durant la période de traitement de 15 semaines
L'entreprise prévoit de faire avancer CM-101 vers un essai de Phase 3 sur la PSC en 2025, après des discussions avec la FDA plus tard cette année.
Chemomab Therapeutics (Nasdaq: CMMB) hat positive Ergebnisse aus seiner Phase-2-SPRING-Studie von CM-101, einem erstmalig eingesetzten monoklonalen Antikörper, bei Patienten mit primärer sklerosierender Cholangitis (PSC) veröffentlicht. Die Studie erfüllte ihren primären Endpunkt hinsichtlich Sicherheit und Verträglichkeit und zeigte anti-fibrotische, anti-entzündliche und anti-cholestatische Effekte über mehrere sekundäre Wirksamkeitsendpunkte.
Wichtige Ergebnisse umfassen:
- Statistisch signifikante Verbesserung der Lebersteifigkeit, eines wichtigen PSC-Krankheitsmarkers
- Verringerung des Gesamtbilirubins, eines wichtigen Indikators für Kolestase und Lebergesundheit
- Verbesserungen bei Juckreiz, einem kolestatischen Indikator, der für Patienten relevant ist
- Günstiges Sicherheitsprofil und allgemeine Verträglichkeit über den 15-wöchigen Behandlungszeitraum
Das Unternehmen plant, CM-101 2025 in eine Phase-3-PSC-Studie zu überführen, nachdem im Laufe dieses Jahres Gespräche mit der FDA geführt wurden.
- CM-101 met the primary endpoint of safety and tolerability in the Phase 2 SPRING trial
- Demonstrated statistically significant improvement in liver stiffness, a key PSC disease marker
- Showed reductions in total bilirubin, an important marker of cholestasis and liver health
- Improved pruritis scores, a relevant indicator for PSC patients
- Exhibited anti-fibrotic, anti-inflammatory, and anti-cholestatic effects across multiple secondary efficacy endpoints
- 90% of eligible study patients elected to join the Open Label Extension portion of the trial
- Plans to advance to Phase 3 PSC trial in 2025
- None.
Insights
The Phase 2 SPRING trial results for CM-101 in Primary Sclerosing Cholangitis (PSC) are highly encouraging. Key findings include:
- CM-101 met its primary endpoint of safety and tolerability
- Demonstrated anti-fibrotic, anti-inflammatory and anti-cholestatic effects
- Statistically significant improvement in liver stiffness, a important PSC disease marker
- Improvements in pruritis, total bilirubin and liver function tests
These results are particularly noteworthy as CM-101 is the first investigational drug for PSC to show broad effects on all three disease components. The statistically significant reduction in liver stiffness after just 15 weeks of treatment is unprecedented in PSC trials. This suggests CM-101's potential to modify disease progression, which could be groundbreaking for PSC patients who currently lack approved treatments.
The dose-dependent responses observed across multiple biomarkers, including ELF score, PRO-C3 and inflammatory cytokines, further support CM-101's mechanism of action. The consistent pattern of greater improvement in patients with moderate/advanced disease is promising for addressing the needs of those at higher risk of rapid disease progression.
However, it's important to note that while these results are promising, they are from a Phase 2 trial. Larger, longer-duration studies will be crucial to confirm CM-101's efficacy and safety profile, especially given PSC's slow progression. The planned Phase 3 trial will be critical in validating these findings and potentially establishing CM-101 as a first-in-class treatment for PSC.
Chemomab Therapeutics' positive Phase 2 results for CM-101 in PSC represent a significant milestone for the company and could have substantial financial implications:
- Market Opportunity: PSC is a rare disease with no approved treatments, presenting a potentially lucrative market for an effective therapy.
- First-Mover Advantage: If approved, CM-101 could be the first-in-class treatment for PSC, potentially commanding premium pricing and capturing significant market share.
- Pipeline Value: The positive results not only de-risk CM-101 for PSC but also validate its potential in other fibrotic diseases, enhancing the overall value of Chemomab's pipeline.
- Partnership Potential: The company's mention of exploring collaboration opportunities could lead to valuable partnerships or licensing deals, potentially providing additional capital and resources.
However, investors should consider several factors:
- Regulatory Path: While promising, CM-101 still needs to succeed in a Phase 3 trial and gain FDA approval, which involves inherent risks and uncertainties.
- Financial Position: As a clinical-stage biotech, Chemomab will likely need additional funding to support a Phase 3 trial and potential commercialization efforts.
- Competition: While CM-101 appears promising, other companies are also developing treatments for PSC and the competitive landscape could evolve.
Overall, these results significantly enhance Chemomab's prospects, but the company's long-term value will depend on successful execution of late-stage clinical development and commercialization strategies.
CM-101's performance in the Phase 2 SPRING trial showcases its potential as a groundbreaking therapy for PSC. The drug's unique mechanism of action, targeting CCL24, appears to address multiple aspects of PSC pathology:
- Anti-fibrotic effects: Demonstrated by improvements in liver stiffness, ELF score and PRO-C3 levels
- Anti-inflammatory effects: Evidenced by reductions in IL-6 and TGFβ1 levels
- Anti-cholestatic effects: Shown through improvements in total bilirubin and liver function tests
This multifaceted approach is particularly promising for a complex disease like PSC. The statistically significant improvement in liver stiffness after just 15 weeks is remarkable, as liver fibrosis is typically slow to respond to treatment.
The dose-dependent responses observed across multiple endpoints suggest a clear pharmacological effect, which bodes well for dose optimization in future studies. The favorable safety profile is also encouraging, especially considering the chronic nature of PSC treatment.
However, it's important to note some limitations:
- The trial duration was relatively short at 15 weeks, given PSC's slow progression
- The sample size of 76 patients, while reasonable for a Phase 2 study, is still small
- Long-term efficacy and safety data will be crucial
The planned Open Label Extension study will provide valuable longer-term data. As CM-101 advances to Phase 3, key considerations will include:
- Optimal dosing regimen
- Patient stratification strategies
- Selection of clinically meaningful endpoints that align with regulatory expectations
Overall, CM-101 represents a promising advance in PSC treatment, with potential applications in other fibrotic diseases. Its success could significantly impact the field of fibrosis research and treatment.
First-in-Class CCL24 Neutralizing Antibody CM-101 Demonstrated a Favorable Safety Profile and Was Generally Well-Tolerated
Demonstrated Anti-Fibrotic, Anti-Inflammatory and Anti-Cholestatic Activity across Multiple Components of Primary Sclerosing Cholangitis (PSC), Establishing Clinical Proof-of-Concept for the Disease-Modifying Potential of CM-101
Treatment with CM-101 Led to Statistically Significant Improvements in Liver Stiffness, a Well-Validated Biomarker of Disease Progression in PSC
Treatment with CM-101 Led to Statistically Significant Improvements in Pruritis and Improvements in Total Bilirubin and Liver Function Tests
Positive Phase 2 Data Paves Way for End-of-Phase 2 Meeting with U.S. Food and Drug Administration and Preparations for a Phase 3 Trial in PSC
Company to Host Conference Call and Webcast Today at 8:00 am ET
TEL AVIV, Israel, July 25, 2024 (GLOBE NEWSWIRE) -- Chemomab Therapeutics Ltd. (Nasdaq: CMMB) (Chemomab) a clinical stage biotechnology company developing innovative therapeutics for fibro-inflammatory diseases with high unmet need, today reported positive topline results from the Phase 2 SPRING trial assessing its first-in-class monoclonal antibody, CM-101, in patients with primary sclerosing cholangitis (PSC). Treatment with CM-101 achieved its primary endpoint of safety and tolerability and demonstrated anti-fibrotic, anti-inflammatory and anti-cholestatic effects across a broad range of disease-related secondary efficacy endpoints, including statistically significant improvement in liver stiffness, a key PSC disease marker. CM-101 is the first investigational drug being developed for PSC to exhibit broad, clinically relevant effects on all three components of the disease, providing further evidence of its multifactorial mechanism of action and disease-modifying potential.
“We are thrilled to report the positive results of the Phase 2 SPRING trial that represent a major milestone for Chemomab and establish clear clinical proof-of-concept for CM-101 in PSC and potentially other fibrotic diseases,” said Adi Mor, PhD, co-founder, Chief Executive Officer and Chief Scientific Officer of Chemomab. “CM-101 achieved the primary and key secondary endpoints in the trial and is the first therapy to demonstrate broad, clinically relevant effects on the three main components of PSC. This is also the first PSC trial to show, after just 15 weeks of treatment, a statistically significant reduction in liver stiffness, a widely used and well validated measure for assessing disease progression in PSC. Moreover, CM-101 is among the first investigational drugs to show a reduction in total bilirubin, an important marker of cholestasis and liver health, as well as reductions in pruritis, a cholestatic indicator of great relevance to patients. We believe these results provide strong support for advancing CM-101 to a Phase 3 PSC trial, which we are planning to initiate in 2025 after our interactions with the FDA later this year. I would like to thank the patients, caregivers and investigators, clinical staff and patient advocacy groups whose commitment and hard work were invaluable.”
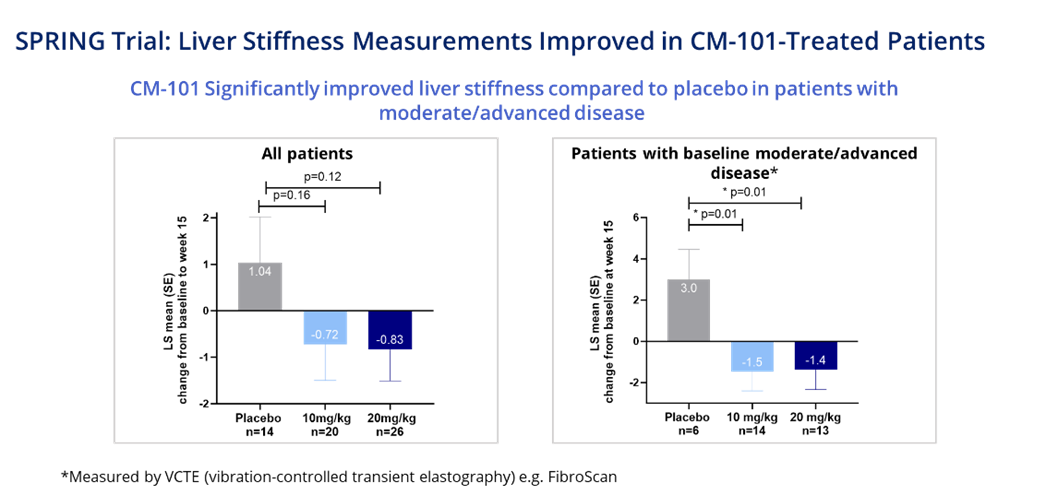
The double-blind placebo-controlled SPRING study assessed two doses of CM-101 (10 mg/kg and 20 mg/kg) administered to PSC patients every three weeks over 15 weeks. A total of 76 patients from the U.S., Europe and Israel were treated in the trial. The study analysis included assessments of all patients who completed all doses and the Week 15 visit, as well as a prespecified subgroup analysis of moderate/advanced patients with a higher risk of more rapidly progressing disease. This population was defined as patients with a vibration-controlled transient elastography (VCTE) measure at baseline of greater than 8.7 kPa. Approximately half of the SPRING study patients were classified as having moderate/advanced disease, which is similar to the overall PSC patient population.
Douglas Thorburn, MD, Divisional Clinical Director for Liver and Digestive Health at the Royal Free London NHS Trust and Professor of Hepatology within the Institute for Liver and Digestive Health at UCL, is Principal Investigator of the SPRING trial. Professor Thorburn said, “It is very encouraging that 15 weeks of treatment with CM-101 for people with PSC was both generally well tolerated and resulted in improvements across such a broad range of disease-related biomarkers, including liver stiffness, ELF, liver enzymes and even pruritis. This is a very positive development for patients with this life-threatening disease that has no approved treatments, and I look forward to the continued clinical progress of CM-101.”
CM-101 SPRING Trial – Overview of Key Results
CM-101 met the primary study endpoint, demonstrating a favorable safety profile over the 15-week treatment period. CM-101-treated patients with moderate/advanced disease showed improvements on a wide range of disease-related secondary endpoints, including assessments of changes from baseline relative to placebo at Week 15 in liver stiffness; in liver fibrosis biomarkers, including the Enhanced Liver Fibrosis (ELF) score and PRO-C3 levels; in total bilirubin and liver function tests; in pruritis (itch) and in markers of inflammation.
Dose-dependent responses were observed for multiple disease-related biomarkers. A consistent pattern of greater improvement on the secondary endpoints was observed in the study arm receiving 20 mg/kg of CM-101 and in the prespecified subgroup of PSC patients with moderate/advanced disease. Since PSC is a slowly progressive disease, longer duration of treatment with CM-101 could potentially result in greater improvement in patient populations with lower disease burden, with the goal of slowing or preventing disease progression.
Primary Endpoint
CM-101 demonstrated a favorable safety profile and was generally well-tolerated over the 15-week treatment period. It also exhibited favorable and dose-dependent pharmacokinetic profiles. Adverse events, which most commonly included fatigue, headache, and pruritis, were generally mild/moderate and distributed similarly between the placebo and CM-101-treated dosing arms.
Key Secondary Endpoints
Liver Stiffness Measures Improved in CM-101-Treated PSC Patients
Notably, both doses of CM-101 improved liver stiffness relative to placebo at Week 15, with a statistically significant improvement achieved in patients with moderate/advanced disease This is the first time that an investigational drug for the treatment of PSC has demonstrated significant improvements in liver stiffness in a relatively short study.
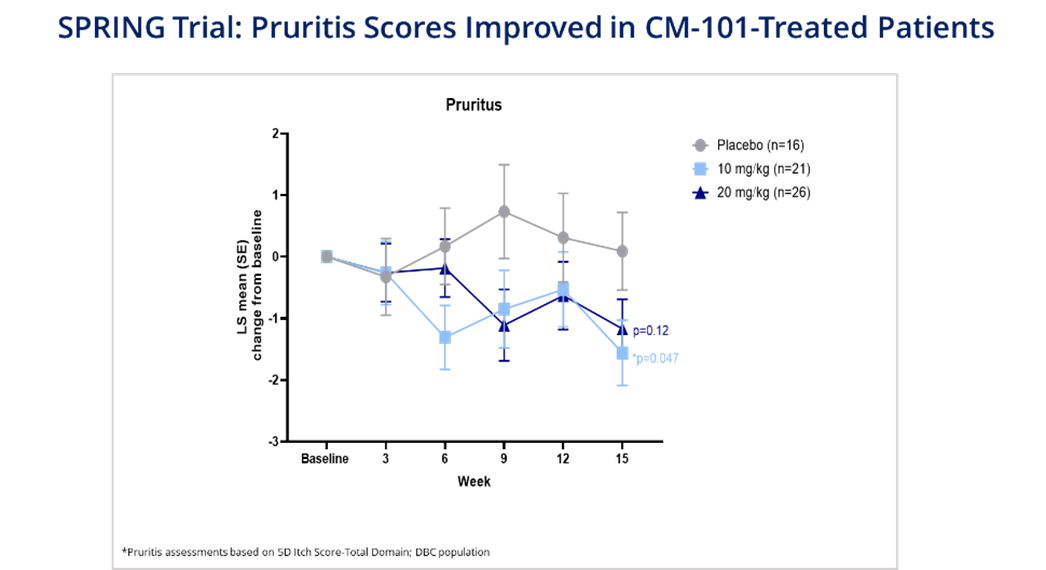
5-D Itch Scale Total Pruritis Scores Improved in CM-101-Treated PSC Patients
Pruritis total scores on the 5-D Itch Scale relative to placebo improved in CM-101-treated patients, who demonstrated decreased pruritis scores compared to placebo starting as soon as six weeks after their first dose. CM-101-treated patients experienced decreased pruritis scores across all timepoints compared to placebo and the decrease reached statistical significance in patients receiving the 10 mg/kg dose at Week 15.
ELF Score Improved in CM-101-Treated PSC Patients
Patients treated with the 20 mg/kg dose of CM-101 with moderate/advanced disease had reduced ELF scores relative to placebo at all time points in the trial. In addition, in all patients treated with the 20 mg/kg dose of CM-101, ELF changes from baseline remained consistently below 0.19, a recognized threshold for predicting long-term PSC-related clinical outcome events.1
PRO-C3 Improved in CM-101-Treated in PSC Patients Reductions in PRO-C3 levels at Week 15 relative to placebo were observed in patients receiving both the 10 mg/kg and 20 mg/kg doses of CM-101. PRO-C3, a serum biomarker of type III collagen synthesis, has been shown to be elevated in patients with PSC and has been identified as an independent predictor of transplant-free survival in PSC.2
Total Bilirubin Improved in CM-101-Treated PSC Patients
Bilirubin is a key biomarker that is an indicator of bile duct health. CM-101-treated patients showed a dose-dependent improvement in total bilirubin relative to placebo at Week 15 that provides further support for the anti-cholestatic activity of CM-101.
Liver Function Tests Improved in CM-101-Treated PSC Patients
All liver function tests improved in CM-101-treated patients relative to placebo at Week 15. Levels of alkaline phosphatase (ALP), alanine aminotransferase (ALT) aspartate aminotransferase (AST) and gamma-glutamyl transferase (GGT) decreased in CM-101-treated patients receiving the 20 mg/kg dose.
Anti-inflammatory Activity Demonstrated in CM-101-Treated PSC Patients
Levels of the inflammatory cytokines IL-6 and TGFβ1, which are well-characterized markers that are downstream from our novel CCL24 target and are known to play an important role in inflammation and fibrosis, were reduced in CM-101-treated patients relative to placebo at Week 15. The reduction reached statistical significance in moderate/advanced disease patients receiving the 20 mg/kg dose.
Christopher Bowlus, MD, the Lena Valente Professor and Chief of the Division of Gastroenterology and Hepatology at the University of California Davis School of Medicine, commented, “These positive trial results come at an opportune time, with promising therapies like CM-101 ready to advance into late-stage trials and recent progress to develop non-invasive biomarkers as registration endpoints in PSC that will meet FDA standards. This is welcome news for the many patients with PSC desperate for new therapeutic options to combat this challenging disease.”
CM-101 in PSC Patients: Next Steps
Chemomab is preparing for an End-of-Phase 2 meeting with the FDA to discuss the SPRING trial results and the design of a proposed Phase 3 PSC trial for accelerated approval. The company anticipates completing these discussions by the end of the year and receiving official written feedback from the FDA in the first quarter of 2025.
An Open Label Extension (OLE) portion of the SPRING trial, which offers patients the opportunity to receive CM-101 for an additional 33-weeks, is ongoing. More than
CM-101’s novel CCL24 target has been of interest to potential partners for several years. The company intends to further explore opportunities to collaborate with strategic partners in light of the positive topline data reported from the SPRING trial.
About the Phase 2 SPRING Trial
Chemomab’s Phase 2 SPRING trial (NCT04595825) is a double-blind, placebo-controlled, multiple dose study assessing the safety and tolerability of CM-101 administered to PSC patients with established large duct disease. The trial treated 76 patients in the U.S., Europe and Israel. Patients received either 10 mg/kg or 20 mg/kg of CM-101 or placebo via an intravenous infusion every three weeks over the 15-week treatment period. The study analysis focused on double-blind completers, defined as participants who received all five doses of CM-101 and had both a baseline and at least one post-baseline measurement of both ALP and ELF. There were 66 patients in the double-blind completer analysis set (50 CM-101-treated patients and 16 placebo patients.) The study analysis plan included a prespecified subgroup of patients with moderate/advanced disease, defined as patients with a VCTE measure at baseline of greater than 8.7 kPa, a well-accepted indicator in PSC of more progressive disease. The SPRING trial includes an OLE that was available to those study participants who completed the double-blind portion once the OLE had been established. OLE participants receive infusions of either 10 mg/kg or 20 mg/kg of CM-101 every three weeks for an additional 33 weeks. In addition to safety, the trial is measuring a wide range of secondary outcomes including serum biomarkers and physiological parameters. These include well-validated liver biomarkers such as assessments of liver stiffness, ELF and PRO-C3, as well as pruritus and liver function tests.
About Primary Sclerosing Cholangitis
PSC is a rare, progressive liver disease characterized by inflammation and fibrosis (scarring) of the bile ducts that can lead to cirrhosis of the liver, liver failure and death. PSC also increases the risk of various cancers, which account for about half of PSC-related mortality. PSC affects an estimated 30,000 patients in the U.S. and about 80,000 worldwide. The underlying cause of PSC is unknown, but about
1 Muir et al, Hepatology, 2019
2 Nielsen et al, Aliment Pharmacol Ther 2018
Conference Call and Webcast
Chemomab management will host a conference call for investors today, Thursday, July 25, 2024, beginning at 8:00 a.m. Eastern Time to discuss the SPRING trial topline results and answer questions.
Click this Webcast link to access the live webcast or replay. The live webcast and replay can also be accessed at the News & Events section of the Investors page on the Chemomab website at investors.chemomab.com/events.
To access the conference call via telephone, shareholders and other interested parties can dial +1 (877) 451-6152 (in the U.S.) or +1 (201) 389-0879 (outside the U.S., including Israel) and enter passcode 13748170. Please call 5-10 minutes before the scheduled start time, enter the conference passcode and ask the operator for the Chemomab conference call.
Or click on Call me™ starting 15 minutes before the scheduled start time for instant telephone access without having to wait for an operator.
Forward Looking Statements
This press release contains forward-looking statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995 that involve substantial risks and uncertainties. All statements other than statements of historical facts contained in this presentation, including statements regarding our future financial condition, results of operations, business strategy and plans, and objectives of management for future operations, as well as statements regarding industry trends, are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “estimate,” “intend,” “may,” “plan,” “potentially” “will” or the negative of these terms or other similar expressions. We have based these forward-looking statements largely on our current expectations and projections about future events and trends that we believe may affect our financial condition, results of operations, business strategy and financial needs. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including, among other things: the risk that the full data set from the CM-101 study or data generated in further clinical trials of CM-101 will not be consistent with the topline results of the CM-101 Phase 2 PSC trial; failure to obtain, or delays in obtaining, regulatory approvals for CM-101 in the U.S., Europe or other territories; failure to successfully commercialize CM-101, if approved by applicable regulatory authorities, in the U.S., Europe or other territories, or to maintain U.S., European or other territory regulatory approval for CM-101 if approved; uncertainties in the degree of market acceptance of CM-101 by physicians, patients, third-party payors and others in the healthcare community; inaccuracies in the Company's estimates of the size of the potential markets for CM-101 or in data the Company has used to identify physicians; expected rates of patient uptake, duration of expected treatment, or expected patient adherence or discontinuation rates; development of unexpected safety or efficacy concerns related to CM-101; failure to successfully conduct future clinical trials for CM-101, including due to the Company's potential inability to enroll or retain sufficient patients to conduct and complete the trials or generate data necessary for regulatory approval, among other things; risks that the Company's clinical studies will be delayed or that serious side effects will be identified during drug development; failure of third parties on which the Company is dependent to manufacture sufficient quantities of CM-101 for commercial or clinical needs, to conduct the Company's clinical trials, or to comply with the Company's agreements or laws and regulations that impact the Company's business or agreements with the Company; the strength and enforceability of the Company’s intellectual property rights or the rights of third parties; the cost and potential reputational damage resulting from litigation to which the Company may become a party, including product liability claims; changes in laws and regulations applicable to the Company's business and failure to comply with such laws and regulations; business or economic disruptions due to catastrophes or other events, including natural disasters or public health crises; and inability to repay the Company's existing indebtedness and uncertainties with respect to the Company's need and ability to access future capital; and the intensity and duration of the current war in Israel, and its impact on our operations in Israel. These risks are not exhaustive. You should carefully consider the risks and uncertainties described in the “Risk Factors” sections of our 20-F for the year ended December 31, 2023. New risk factors emerge from time to time, and it is not possible for our management to predict all risk factors, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in, or implied by, any forward-looking statements. You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. Except as required by law, we undertake no obligation to update publicly any forward-looking statements for any reason after the date of this presentation. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy these securities, nor shall there be any sale of these securities in any state or jurisdiction in which such offer, solicitation, or sale would be unlawful prior to registration or qualification under the securities law of any such state or jurisdiction.
About Chemomab Therapeutics Ltd.
Chemomab is a clinical stage biotechnology company developing innovative therapeutics for fibro-inflammatory diseases with high unmet need. Based on the unique and pivotal role of CCL24 in promoting fibrosis and inflammation, Chemomab developed CM-101, a monoclonal antibody that neutralizes CCL24 activity. In clinical and preclinical studies, CM-101 has been shown to have a favorable safety profile and has been generally well-tolerated to date, with the potential to treat multiple severe and life-threatening fibro-inflammatory diseases. Chemomab has reported positive results from four clinical trials of CM-101, including a Phase 2 trial in patients with primary sclerosing cholangitis, a Phase 2a liver fibrosis trial in patients with metabolic-dysfunction associated-steatohepatitis, a Phase 1b study in patients with metabolic dysfunction–associated fatty liver disease and an investigator-initiated study in patients with severe lung injury. Chemomab’s CM-101 program for the treatment of systemic sclerosis is Phase 2-ready with an open U.S. IND. For more information about Chemomab, visit chemomab.com.
Contact:
Media & Investors:
Chemomab Therapeutics
Barbara Lindheim
Consulting Vice President
Investor & Public Relations, Strategic Communications
Phone: +1 917-355-9234
barbara.lindheim@chemomab.com
IR@chemomab.com
Photos accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/97e9dd42-3978-4192-b1aa-dd84c7948079
https://www.globenewswire.com/NewsRoom/AttachmentNg/62f9cf98-b5da-4673-ae5a-931592670482








