Can-Fite to Generate $685M in Projected Future Revenues from its Partnerships
Can-Fite BioPharma (NYSE: CANF) has announced projected future revenues of $685M from its partnerships, based on a comprehensive analysis of its current partnerships and market potential for lead drug candidates Piclidenoson and Namodenoson.
The revenue forecast spans the next decade and covers four key indications: psoriasis, advanced liver cancer, pancreatic cancer, and MASH. The projection assumes regulatory approvals and product launches between 2027 and 2029, varying by indication and territory.
The company's seven partnerships include various revenue streams such as:
- Development and regulatory milestones
- Commercial sales benchmarks
- Manufacturing-related transfer payments
- Royalties on product sales
Can-Fite BioPharma (NYSE: CANF) ha annunciato entrate future previste di $685 milioni derivanti dalle sue partnership, basate su un'analisi approfondita delle attuali collaborazioni e del potenziale di mercato per i principali candidati farmaceutici Piclidenoson e Namodenoson.
La previsione di entrate si estende per il prossimo decennio e copre quattro indicazioni chiave: psoriasi, cancro al fegato avanzato, cancro pancreatico e MASH. La proiezione presuppone approvazioni regolatorie e lanci di prodotto tra il 2027 e il 2029, variando in base all'indicazione e al territorio.
Le sette partnership dell'azienda includono varie fonti di reddito come:
- Traguardi nello sviluppo e nella regolamentazione
- Obiettivi di vendita commerciale
- Pagamenti di trasferimento legati alla produzione
- Royalty sulle vendite di prodotti
Can-Fite BioPharma (NYSE: CANF) ha anunciado ingresos futuros proyectados de $685 millones provenientes de sus asociaciones, basados en un análisis exhaustivo de sus asociaciones actuales y el potencial de mercado para los principales candidatos a medicamentos Piclidenoson y Namodenoson.
La previsión de ingresos abarca la próxima década y cubre cuatro indicaciones clave: psoriasis, cáncer de hígado avanzado, cáncer de páncreas y MASH. La proyección asume aprobaciones regulatorias y lanzamientos de productos entre 2027 y 2029, variando según la indicación y el territorio.
Las siete asociaciones de la empresa incluyen diversas fuentes de ingresos como:
- Hitos de desarrollo y regulatorios
- Referencias de ventas comerciales
- Pagos de transferencia relacionados con la fabricación
- Regalías sobre las ventas de productos
Can-Fite BioPharma (NYSE: CANF)는 현재의 파트너십과 주요 약물 후보인 Piclidenoson 및 Namodenoson의 시장 잠재력에 대한 포괄적인 분석을 바탕으로 $685M의 미래 예상 수익을 발표했습니다.
수익 예측은 향후 10년에 걸쳐 있으며, 건선, 진행성 간암, 췌장암 및 MASH의 네 가지 주요 적응증을 포함합니다. 이 예측은 2027년과 2029년 사이에 적응증 및 지역에 따라 달라지는 규제 승인 및 제품 출시를 가정합니다.
회사의 7개 파트너십에는 다음과 같은 다양한 수익원이 포함됩니다:
- 개발 및 규제 이정표
- 상업적 판매 기준
- 제조 관련 이전 지불
- 제품 판매에 대한 로열티
Can-Fite BioPharma (NYSE: CANF) a annoncé des revenus futurs projetés de 685 millions de dollars issus de ses partenariats, sur la base d'une analyse complète de ses partenariats actuels et du potentiel de marché de ses principaux candidats médicaments Piclidenoson et Namodenoson.
La prévision de revenus s'étend sur la prochaine décennie et couvre quatre indications clés : psoriasis, cancer du foie avancé, cancer du pancréas et MASH. La projection suppose des approbations réglementaires et des lancements de produits entre 2027 et 2029, variant selon l'indication et le territoire.
Les sept partenariats de l'entreprise comprennent diverses sources de revenus telles que :
- Jalons de développement et réglementaires
- Critères de vente commerciale
- Paiements de transfert liés à la fabrication
- Redevances sur les ventes de produits
Can-Fite BioPharma (NYSE: CANF) hat zukünftige Einnahmen in Höhe von 685 Millionen US-Dollar aus seinen Partnerschaften angekündigt, basierend auf einer umfassenden Analyse seiner aktuellen Partnerschaften und des Marktpotenzials für die Hauptmedikamentenkandidaten Piclidenoson und Namodenoson.
Die Umsatzprognose erstreckt sich über das nächste Jahrzehnt und umfasst vier wichtige Indikationen: Psoriasis, fortgeschrittenen Leberkrebs, Bauchspeicheldrüsenkrebs und MASH. Die Prognose geht von behördlichen Genehmigungen und Produkteinführungen zwischen 2027 und 2029 aus, je nach Indikation und Gebiet.
Die sieben Partnerschaften des Unternehmens umfassen verschiedene Einnahmequellen wie:
- Entwicklungs- und regulatorische Meilensteine
- Kommerzielle Verkaufsbenchmarks
- Herstellungsbezogene Überweisungszahlungen
- Lizenzgebühren auf Produktverkäufe
- Projected substantial revenue of $685M from partnerships
- Diversified revenue streams from seven partnership agreements
- Multiple drug candidates targeting four different medical conditions
- Clear timeline for potential product launches (2027-2029)
- Revenue projections are speculative and depend on future regulatory approvals
- No current revenue generation from these partnerships
- Product launches are 2-4 years away
- Success depends on clinical trials outcomes and market penetration
Insights
Can-Fite's projection of
The announcement lacks critical details that would enhance its credibility, including the projected timeframe for realizing the
While partnership-based revenue models offer advantages through risk-sharing and upfront capital, the structure means Can-Fite likely receives only a fraction of end-market sales. The diversified revenue streams (development/regulatory milestones, sales benchmarks, manufacturing payments, and royalties) provide multiple potential catalysts but also dependency on partners' execution capabilities.
The company's acknowledgment of "inherent uncertainties" serves as an important qualifier, effectively recognizing the high failure rates in biotech drug development. Without specifics on clinical progress or remaining developmental hurdles, investors have basis for assessing likelihood of achievement beyond the company's internal projections.
Can-Fite's dual-asset strategy targeting four significant disease indications represents a potentially valuable approach in biotech development. Piclidenoson and Namodenoson address substantial markets in inflammatory (psoriasis) and oncology (liver/pancreatic cancers) indications, plus MASH (Metabolic dysfunction-Associated SteatoHepatitis), which represents a large untapped therapeutic area with substantial unmet need.
The projected 2027-2029 approval timeline suggests mid-to-late stage clinical development for these assets. However, the announcement conspicuously omits current clinical trial phases and results, which are critical determinants of approval probability. Regulatory success rates vary dramatically between Phase 2 (
The company's seven partnership structure indicates significant external validation of their technology platform and development approach. Multiple partners diversify risk while potentially accelerating market penetration across different territories post-approval. However, partnership-dependent commercialization also means relinquishing significant control over development pace and marketing strategy.
Without specific data on current clinical outcomes, regulatory interactions, or competitive positioning within each indication, these revenue projections remain highly speculative. The extended timeline to commercialization also leaves substantial opportunity for competitive entries and evolving treatment paradigms that could materially impact market potential upon eventual approval.
Ramat Gan, Israel, April 14, 2025 (GLOBE NEWSWIRE) -- Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF), a biotechnology company advancing a pipeline of proprietary small-molecule drugs for oncological and inflammatory diseases, today announced the completion of a comprehensive analysis of its current partnerships and the market potential for its lead drug candidates, Piclidenoson and Namodenoson upon regulatory approvals.
Based on in-depth internal modeling and insights from external advisors, the Company forecasts potential substantial revenue generation over the next decade from its two drug candidates, currently in development for four key indications: psoriasis, advanced liver cancer, pancreatic cancer, and MASH, which assumes, among other things, regulatory approval and launches between 2027 and 2029, depending on the indication and territory. Can-Fite’s seven partnerships are structured with diverse financial components, including development and regulatory milestones, commercial sales benchmarks, manufacturing-related transfer payments, and royalties on product sales. Integrating these partnership terms into its projections, the Company anticipates potential significant cumulative income from multiple revenue streams, reinforcing its potential long-term growth prospects based on assumed achievement of milestones, regulatory approval, launches, market penetration and market size.
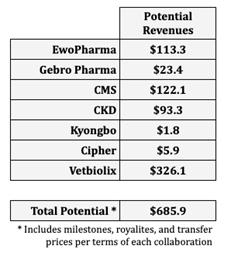
“While we emphasize these figures are derived from our forecasts and subject to inherent uncertainties of drug development and commercialization, we remain highly encouraged by these projections. They underscore the robust strategic foundation that we have built through our diverse collaborations, reflecting both the significant commercial opportunities and potential long-term value we aim to deliver to our shareholders”, stated Can-Fite VP Business Development Dr. Sari Fishman.
About Can-Fite BioPharma Ltd.
Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF) is an advanced clinical stage drug development Company with a platform technology that is designed to address multi-billion dollar markets in the treatment of cancer, liver, and inflammatory disease. The Company’s lead drug candidate, Piclidenoson recently reported topline results in a Phase III trial for psoriasis and commenced a pivotal Phase III trial. Can-Fite’s liver drug, Namodenoson, is being evaluated in a Phase III trial for hepatocellular carcinoma (HCC), a Phase IIb trial for the treatment of MASH, and in a Phase IIa study in pancreatic cancer. Namodenoson has been granted Orphan Drug Designation in the U.S. and Europe and Fast Track Designation as a second line treatment for HCC by the U.S. Food and Drug Administration. Namodenoson has also shown proof of concept to potentially treat other cancers including colon, prostate, and melanoma. CF602, the Company’s third drug candidate, has shown efficacy in the treatment of erectile dysfunction. These drugs have an excellent safety profile with experience in over 1,600 patients in clinical studies to date. For more information please visit: www.canfite.com.
Forward-Looking Statements
This press release may contain forward-looking statements, about Can-Fite’s expectations, beliefs or intentions regarding, among other things, its revenue projections and prospects over the next decade. All statements in this communication, other than those relating to historical facts, are “forward looking statements”. Forward-looking statements can be identified by the use of forward-looking words such as “believe,” “expect,” “intend,” “plan,” “may,” “should” or “anticipate” or their negatives or other variations of these words or other comparable words or by the fact that these statements do not relate strictly to historical or current matters. Forward-looking statements relate to anticipated or expected events, activities, trends or results as of the date they are made. Because forward-looking statements relate to matters that have not yet occurred, these statements are inherently subject to known and unknown risks, uncertainties and other factors that may cause Can-Fite’s actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Important factors that could cause actual results, performance or achievements to differ materially from those anticipated in these forward-looking statements include, among other things, our history of losses and needs for additional capital to fund our operations and our inability to obtain additional capital on acceptable terms, or at all; uncertainties of cash flows and inability to meet working capital needs; the initiation, timing, progress and results of our preclinical studies, clinical trials and other product candidate development efforts; our ability to advance our product candidates into clinical trials or to successfully complete our preclinical studies or clinical trials; our receipt of regulatory approvals for our product candidates, and the timing of other regulatory filings and approvals; the clinical development, commercialization and market acceptance of our product candidates; our ability to establish and maintain strategic partnerships and other corporate collaborations; the implementation of our business model and strategic plans for our business and product candidates; the scope of protection we are able to establish and maintain for intellectual property rights covering our product candidates and our ability to operate our business without infringing the intellectual property rights of others; competitive companies, technologies and our industry; risks related to not satisfying the continued listing requirements of NYSE American; and statements as to the impact of the political and security situation in Israel on our business. More information on these risks, uncertainties and other factors is included from time to time in the “Risk Factors” section of Can-Fite’s Annual Report on Form 20-F filed with the SEC on April 14, 2025 and other public reports filed with the SEC and in its periodic filings with the TASE. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. Can-Fite undertakes no obligation to publicly update or review any forward-looking statement, whether as a result of new information, future developments or otherwise, except as may be required by any applicable securities laws.
Contact
Can-Fite BioPharma
Motti Farbstein
+972-3-9241114








