BeyondSpring Delivers Oral Presentation at ISLAC 2024 World Conference on Lung Cancer of its Lead Anti-Cancer Asset Plinabulin Showcasing Positive Final Phase 3 Data in 2L/3L NSCLC EGFR Wild-Type with Concurrent Publication in the Lancet Respiratory Medicine
BeyondSpring Inc. (NASDAQ: BYSI) presented final Phase 3 data of its lead anti-cancer asset Plinabulin at ISLAC 2024 World Conference on Lung Cancer. The DUBLIN-3 study showed statistically significant and clinically meaningful efficacy data for Plinabulin plus Docetaxel vs. Docetaxel alone in EGFR wild-type NSCLC patients after progression on platinum-based therapy. Key findings include:
- Improved overall survival (OS) with HR=0.82
- Better progression-free survival (PFS) with HR=0.79
- Nearly doubled objective response rate (ORR)
- Doubled 24-month and 36-month OS rates
- 82% reduction in Grade 4 neutropenia
The combination showed a favorable benefit/risk ratio and could be considered as a new treatment option for this population with high unmet medical needs.
BeyondSpring Inc. (NASDAQ: BYSI) ha presentato i dati finali della Fase 3 del suo principale asset anti-cancro Plinabulin alla Conferenza Mondiale sul Cancro Polmonare ISLAC 2024. Lo studio DUBLIN-3 ha mostrato dati di efficacia statisticamente significativi e clinicamente rilevanti per Plinabulin in combinazione con Docetaxel rispetto a Docetaxel da solo nei pazienti con NSCLC a tipo selvaggio EGFR dopo progressione su terapia a base di platino. I risultati chiave includono:
- Improvemento della sopravvivenza globale (OS) con HR=0.82
- Migliore sopravvivenza libera da progressione (PFS) con HR=0.79
- Quasi raddoppiato il tasso di risposta obiettiva (ORR)
- Doppio tasso di OS a 24 e 36 mesi
- Riduzione del 82% della neutropenia di grado 4
La combinazione ha mostrato un rapporto beneficio/rischio favorevole e potrebbe essere considerata come una nuova opzione terapeutica per questa popolazione con elevate esigenze mediche insoddisfatte.
BeyondSpring Inc. (NASDAQ: BYSI) presentó los datos finales de la Fase 3 de su principal activo anti-cáncer Plinabulin en la Conferencia Mundial sobre Cáncer de Pulmón ISLAC 2024. El estudio DUBLIN-3 mostró datos de eficacia estadísticamente significativos y clínicamente relevantes para Plinabulin más Docetaxel frente a Docetaxel solo en pacientes con NSCLC tipo salvaje EGFR después de la progresión en terapia a base de platino. Los hallazgos clave incluyen:
- Mejora de la supervivencia global (OS) con HR=0.82
- Mejor supervivencia libre de progresión (PFS) con HR=0.79
- Casi duplicado el índice de respuesta objetiva (ORR)
- Doblado las tasas de OS a 24 y 36 meses
- Reducción del 82% en neutropenia de grado 4
La combinación mostró una relación beneficio/riesgo favorable y podría considerarse como una nueva opción de tratamiento para esta población con altas necesidades médicas insatisfechas.
BeyondSpring Inc. (NASDAQ: BYSI)는 ISLAC 2024 세계 폐암 회의에서 주요 항암 자산인 Plinabulin의 최종 3상 데이터를 발표했습니다. DUBLIN-3 연구는 백금 기반 치료 후 진행한 EGFR 야생형 NSCLC 환자에서 Docetaxel 단독 대비 Plinabulin과 Docetaxel의 조합이 통계적으로 유의미하고 임상적으로 의미 있는 효능 데이터를 보여주었습니다. 주요 발견 사항은 다음과 같습니다:
- 전체 생존율(OS) 개선, HR=0.82
- 진행 없는 생존율(PFS) 개선, HR=0.79
- 목표 반응률(ORR)이 거의 두 배 증가
- 24개월 및 36개월 OS 비율 두 배 증가
- 4등급 호중구감소증에서 82% 감소
이 조합은 유리한 이익/위험 비율을 보여주며, 높은 의료적 요구가 있는 인구를 위한 새로운 치료 옵션으로 고려될 수 있습니다.
BeyondSpring Inc. (NASDAQ: BYSI) a présenté les données finales de la phase 3 de son principal actif anticancéreux Plinabulin lors de la conférence mondiale sur le cancer du poumon ISLAC 2024. L'étude DUBLIN-3 a montré des données d'efficacité statistiquement significatives et cliniquement pertinentes pour Plinabulin associé à Docetaxel par rapport à Docetaxel seul chez les patients atteints de NSCLC de type sauvage EGFR après progression sous traitement à base de platine. Les principales conclusions incluent :
- Amélioration de la survie globale (OS) avec HR=0.82
- Meilleure survie sans progression (PFS) avec HR=0.79
- Taux de réponse objective (ORR) presque doublé
- Taux de survie à 24 et 36 mois doublés
- Réduction de 82 % de la neutropénie de grade 4
La combinaison a montré un rapport bénéfice/risque favorable et pourrait être considérée comme une nouvelle option de traitement pour cette population ayant des besoins médicaux non satisfaits élevés.
BeyondSpring Inc. (NASDAQ: BYSI) hat die endgültigen Phase-3-Daten seines Haupt-Antikrebsmedikaments Plinabulin auf der ISLAC 2024 Weltkonferenz über Lungenkrebs vorgestellt. Die DUBLIN-3-Studie zeigte statistisch signifikante und klinisch bedeutsame Effektivitätsdaten von Plinabulin plus Docetaxel gegenüber Docetaxel allein bei Patienten mit EGFR Wildtyp NSCLC nach Progression unter einer auf Platinen basierenden Therapie. Die wichtigsten Ergebnisse umfassen:
- Verbesserte Gesamtüberlebensrate (OS) mit HR=0.82
- Bessere progressionsfreie Überlebensrate (PFS) mit HR=0.79
- Beinahe verdoppelte objektive Ansprechraten (ORR)
- Verdoppelte OS-Raten bei 24 und 36 Monaten
- 82% Reduktion der Grade-4-Neutropenie
Die Kombination zeigte ein günstiges Nutzen-Risiko-Verhältnis und könnte als neue Behandlungsoption für diese Bevölkerung mit hohen unerfüllten medizinischen Bedürfnissen in Betracht gezogen werden.
- Statistically significant improvement in overall survival (OS) with HR=0.82
- Better progression-free survival (PFS) with HR=0.79
- Nearly doubled objective response rate (ORR)
- Doubled 24-month and 36-month OS rates
- 82% reduction in Grade 4 neutropenia
- Consistent OS benefit in 24-month follow-up after database lock
- Improved OS benefit with more cycles of treatment (HR=0.64 for ≥4 cycles)
- Well-tolerated combination therapy
- Higher incidences of Grade 3/4 gastrointestinal disorders in Plinabulin group (16.8% vs 2.9%)
- Increased Grade 3 hypertension in Plinabulin group (18.2% vs 2.9%)
Insights
The DUBLIN-3 phase 3 study results for plinabulin in combination with docetaxel are highly significant for 2L/3L NSCLC treatment. Key findings include:
- Statistically significant improvement in overall survival (OS) with HR=0.82
- Enhanced progression-free survival (PFS) with HR=0.79
- Nearly doubled objective response rate (ORR)
82% reduction in Grade 4 neutropenia
These results are particularly impressive given that six recent phase 3 studies failed to show OS benefits in this population. The consistent OS benefit in 24-month follow-up and improved outcomes with more treatment cycles suggest durable efficacy. The combination's well-tolerated safety profile further enhances its potential as a new standard of care for this challenging patient group.
The plinabulin/docetaxel combination shows promising results for EGFR wild-type NSCLC patients who've progressed on platinum-based therapy. The 2.6-month median OS benefit in non-squamous patients is clinically meaningful. Importantly, patients receiving ≥4 treatment cycles showed an impressive OS HR of 0.64 (p=0.0027), with a 4.8-month mOS benefit. This suggests that longer treatment duration could significantly improve outcomes. The reduction in Grade 4 neutropenia is also crucial, as it could improve quality of life and potentially allow for more consistent dosing. However, the increased incidence of Grade 3 GI disorders and hypertension warrants careful patient monitoring and management.
The positive DUBLIN-3 results could significantly impact BeyondSpring's market position. With plinabulin showing efficacy where other recent trials have failed, it has the potential to capture a substantial share of the 2L/3L NSCLC market. The concurrent publication in Lancet Respiratory Medicine adds credibility and visibility. Key financial implications include:
- Potential for accelerated regulatory approvals
- Increased interest from potential partners or acquirers
- Possible expansion into first-line treatment or combination with immunotherapies
Investors should monitor upcoming regulatory interactions and any partnership discussions. While the news is positive, commercialization challenges and competition from established players could impact future revenue potential.
- Statistically Significant and Clinically Meaningful Efficacy Data with Favorable Benefit/Risk ratio from DUBLIN-3 Phase 3 Study of Plinabulin plus Docetaxel vs. Docetaxel in EGFR Wild-Type NSCLC after Progression on Platinum-based Therapy
- Plinabulin/Docetaxel Combination Significantly Improved Overall Survival (OS), Progression Free Survival (PFS), Objective Response Rate (ORR), 2- and 3-year OS Rates and Significantly Reduced Grade 4 Neutropenia vs. Docetaxel
FLORHAM PARK, N.J., Sept. 10, 2024 (GLOBE NEWSWIRE) -- BeyondSpring Inc. (NASDAQ: BYSI) (“BeyondSpring” or the “Company”), a clinical-stage global biopharmaceutical company focused on developing innovative cancer therapies, today announces Dublin-3 final phase 3 efficacy data of its late clinical-stage agent Plinabulin in combination with docetaxel in second/third line (2L/3L) advanced and metastatic non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) wild-type. The lead principal investigator, Dr. Trevor Feinstein from Piedmont Cancer Institute, presented the data in an oral presentation (OA08.04) at ISLAC 2024 World Conference on Lung Cancer on September 9th 2024 in San Diego, CA. Concurrently, Dublin-3 study was published in the Lancet Respiratory Medicine (https://doi.org/10.1016/S2213-2600(24)00178-4).
Docetaxel remains the standard of care for patients with 2L/3L NSCLC without targetable alterations despite severe neutropenia (>
The DUBLIN-3 study was a multicenter, randomized, single-blind, placebo-controlled trial that enrolled patients from 58 medical centers internationally. For the intention-to-treat (ITT) population, 559 patients received either docetaxel plus plinabulin (n=278 [male 199, female 79]) or docetaxel plus placebo (n=281 [male 207, female 74]).
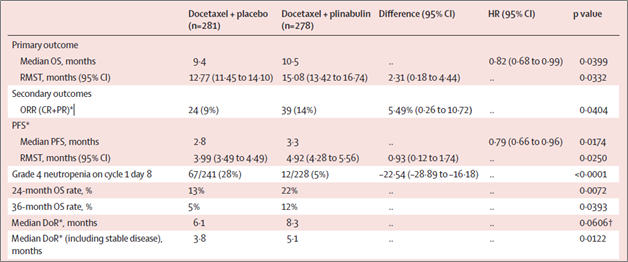
Key findings of Dublin-3 study are summarized below:
- Favorable benefit/risk ratio: Significant improvement in OS (Hazard ratio or HR=0.82; same HR in the Western vs. Asian patients), PFS (HR=0.79) and ORR (nearly doubled). Durable anti-cancer benefits in doubling 24-months and 36-months OS rates. And
82% relative reduction in Grade 4 neutropenia in Cycle 1 Day 8 (p<0.0001). - Consistent OS benefit in 24-month follow up after the database lock: OS HR=0.81 in the ITT population, with better OS benefit in the non-squamous subset (OS HR=0.72, p=0.0078). For the non-squamous subset patients, median OS (mOS) in plinabulin/docetaxel arm was 11.4 months vs. 8.8 months in the docetaxel arm, with a mOS benefit of 2.6 months.
- Improved OS benefit with more cycles of treatment (≥ 4, 6, 8, 10, or 12 cycles): for patients who used at least 4 cycles of treatment, OS HR=0.64, p=0.0027, with a mOS benefit of 4.8 months (plinabulin/docetaxel arm n=133; docetaxel arm n=127).
- Plinabulin/docetaxel combination is well-tolerated: Treatment-emergent adverse-events occurred in 273/274 (99·
6% ) of patients in the plinabulin group and 276/278 (99·3% ) in the control group. Higher incidences of Grade 3/4 gastrointestinal disorders (46 patients [16·8% ] vs. 8 [2·9% ]) and transient Grade 3 hypertension (50 patients [18·2% ] vs. 8 [2·9% ]) occurred in the plinabulin vs. control group.
“There is a poor prognosis for NSCLC patients without targetable alterations whose disease has progressed on platinum-based therapies. Unfortunately, multiple high-profile phase 3 studies failed to show overall survival benefit in this hard-to-treat population compared to standard of care docetaxel, a drug approved over 20 years ago. The data from DUBLIN-3 study demonstrates that the addition and proper sequencing of plinabulin to docetaxel has a favorable benefit/risk ratio compared with docetaxel alone and may have broad utility. This combination could be considered as a new treatment option for this population with high unmet medical needs,” said Dr. Feinstein, a lead principal investigator of the DUBLIN-3 study at Piedmont Cancer Center, Atlanta.
Citation:
Han B., et al. Plinabulin plus docetaxel versus docetaxel in patients with non-small-cell lung cancer after disease progression on platinum-based regimen (DUBLIN-3): a phase 3, international, multicentre, single-blind, parallel group, randomised controlled trial. Lancet Respir Med. 2024 Sep 09: S2213-2600(24)00178-4.
About Plinabulin
Plinabulin is a novel first-in-class dendritic cell maturation agent with durable anti-cancer benefit observed across multiple clinical studies. As a reversible binder at a distinct tubulin pocket, plinabulin does not change tubulin dynamics or antagonize tubulin stabilizing agents, such as docetaxel, which contributes to its differentiated activity and tolerability compared to other tubulin binders. In addition, plinabulin significantly reduces chemotherapy induced neutropenia and could thereby increase docetaxel tolerability. Over 700 patients have been treated with plinabulin with good tolerability.
About Dublin-3 Study
DUBLIN-3 is a multicenter, single-blinded (patient) and randomized, phase 3 trial in 58 medical centers (US, China, and Australia). Only patients with EGFR wild-type NSCLC who had progressed after first-line platinum-based therapy were enrolled. Patients were randomized (1:1) to receive docetaxel (75 mg/m2) on Day 1 and either plinabulin (30 mg/m2) or placebo on Days 1 and 8 in 21-day cycles until progression, unacceptable toxicity, withdrawal, or death. Treated patients were included in the safety analysis and ITT population in the primary efficacy analyses (NCT02504489). The primary endpoint for the study was OS, and secondary endpoints were PFS, ORR, Duration of Response (DoR), Grade 4 neutropenia and Quality of Life.
About BeyondSpring
BeyondSpring is a global clinical-stage biopharmaceutical company focused on developing innovative therapies to improve clinical outcomes for patients with high unmet medical needs. The Company is advancing its first-in-class lead asset, Plinabulin, a potent inducer of dendritic cell maturation, in late-stage clinical development as a direct anti-cancer agent in NSCLC and a variety of cancer indications. BeyondSpring’s pipeline also includes three preclinical immuno-oncology assets. Additionally, BeyondSpring is an equity owner of SEED Therapeutics, Inc which is a pioneer in Target Protein Degradation technology and its application in innovative drug development. Learn more by visiting https://beyondspringpharma.com.
Investor Contact:
IR@beyondspringpharma.com
Media Contact:
PR@beyondspringpharma.com
A photo accompanying this announcement is available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/66242c15-b61a-48be-a69d-8cf334951a84








