Alzamend Neuro Announces Initiation Date of Phase II Clinical Trial of AL001 for Treatment of Major Depressive Disorder to take Place at Massachusetts General Hospital
Alzamend Neuro (NASDAQ: ALZN) has announced plans to initiate a Phase II clinical trial of AL001 for Major Depressive Disorder (MDD) treatment in Q4 2025 at Massachusetts General Hospital. The study follows the successful completion of a head coil by Tesla Dynamic Coils BV.
The trial will compare AL001 with marketed lithium carbonate, focusing on lithium blood and brain pharmacokinetics in MDD patients. Previous mouse studies showed AL001 achieves better brain absorption while maintaining lower blood lithium levels, potentially eliminating the need for therapeutic drug monitoring (TDM).
AL001 aims to overcome limitations of current FDA-approved lithium salts, which require regular monitoring due to a narrow therapeutic window. While lithium lacks FDA approval for MDD treatment, it has been prescribed off-label for decades as an antidepressant augmentation therapy, showing efficacy in multiple randomized controlled trials.
Alzamend Neuro (NASDAQ: ALZN) ha annunciato piani per avviare uno studio clinico di Fase II di AL001 per il trattamento del Disturbo Depressivo Maggiore (MDD) nel quarto trimestre del 2025 presso il Massachusetts General Hospital. Lo studio segue il completamento con successo di una bobina per la testa da parte di Tesla Dynamic Coils BV.
Il trial confronterà AL001 con il carbonato di litio commercializzato, concentrandosi sulla farmacocinetica del litio nel sangue e nel cervello nei pazienti con MDD. Studi precedenti sui topi hanno mostrato che AL001 raggiunge una migliore assorbimento cerebrale mantenendo livelli di litio nel sangue più bassi, potenzialmente eliminando la necessità di monitoraggio terapeutico dei farmaci (TDM).
AL001 mira a superare le limitazioni dei sali di litio attualmente approvati dalla FDA, che richiedono un monitoraggio regolare a causa di una finestra terapeutica ristretta. Sebbene il litio non abbia l'approvazione della FDA per il trattamento del MDD, è stato prescritto off-label per decenni come terapia di potenziamento antidepressivo, mostrando efficacia in numerosi studi controllati randomizzati.
Alzamend Neuro (NASDAQ: ALZN) ha anunciado planes para iniciar un ensayo clínico de Fase II de AL001 para el tratamiento del Trastorno Depresivo Mayor (MDD) en el cuarto trimestre de 2025 en el Massachusetts General Hospital. El estudio sigue la exitosa finalización de una bobina para la cabeza por parte de Tesla Dynamic Coils BV.
El ensayo comparará AL001 con el carbonato de litio comercializado, centrándose en la farmacocinética del litio en sangre y cerebro en pacientes con MDD. Estudios previos en ratones mostraron que AL001 logra una mejor absorción cerebral mientras mantiene niveles de litio en sangre más bajos, lo que potencialmente elimina la necesidad de monitoreo terapéutico de medicamentos (TDM).
AL001 tiene como objetivo superar las limitaciones de las sales de litio aprobadas por la FDA, que requieren un monitoreo regular debido a una ventana terapéutica estrecha. Aunque el litio no tiene aprobación de la FDA para el tratamiento del MDD, se ha prescrito off-label durante décadas como terapia de aumento antidepresiva, mostrando eficacia en múltiples ensayos controlados aleatorios.
Alzamend Neuro (NASDAQ: ALZN)는 2025년 4분기에 매사추세츠 종합병원에서 MDD(주요 우울 장애) 치료를 위한 AL001의 2상 임상 시험을 시작할 계획을 발표했습니다. 이 연구는 Tesla Dynamic Coils BV에 의해 머리 코일이 성공적으로 완공된 후 진행됩니다.
시험은 AL001과 시판 중인 탄산리튬을 비교하며, MDD 환자에서 리튬의 혈액 및 뇌 약동학에 초점을 맞춥니다. 이전 쥐 연구에서는 AL001이 더 낮은 혈중 리튬 수치를 유지하면서 더 나은 뇌 흡수를 달성한다는 결과가 나왔으며, 이는 치료 약물 모니터링(TDM)의 필요성을 없앨 수 있습니다.
AL001은 정기적인 모니터링이 필요한 FDA 승인 리튬염의 한계를 극복하는 것을 목표로 합니다. 리튬은 MDD 치료를 위한 FDA 승인을 받지 않았지만, 수십 년 동안 항우울제 보강 요법으로 오프라벨로 처방되어 왔으며, 여러 무작위 대조 시험에서 효능을 보여주었습니다.
Alzamend Neuro (NASDAQ: ALZN) a annoncé des projets pour initier un essai clinique de Phase II d'AL001 pour le traitement du Trouble Dépressif Majeur (MDD) au quatrième trimestre 2025 au Massachusetts General Hospital. L'étude fait suite à l'achèvement réussi d'une bobine pour la tête par Tesla Dynamic Coils BV.
L'essai comparera AL001 avec le carbonate de lithium commercialisé, en se concentrant sur la pharmacocinétique du lithium dans le sang et le cerveau chez les patients atteints de MDD. Des études antérieures sur des souris ont montré qu'AL001 atteint une meilleure absorption cérébrale tout en maintenant des niveaux de lithium sanguin plus bas, ce qui pourrait éliminer la nécessité d'un suivi thérapeutique des médicaments (TDM).
AL001 vise à surmonter les limitations des sels de lithium actuellement approuvés par la FDA, qui nécessitent un suivi régulier en raison d'une fenêtre thérapeutique étroite. Bien que le lithium n'ait pas l'approbation de la FDA pour le traitement du MDD, il a été prescrit hors étiquette pendant des décennies en tant que thérapie d'augmentation des antidépresseurs, montrant une efficacité dans plusieurs essais contrôlés randomisés.
Alzamend Neuro (NASDAQ: ALZN) hat Pläne angekündigt, im vierten Quartal 2025 eine Phase-II-Studie zu AL001 zur Behandlung der Major Depression (MDD) im Massachusetts General Hospital zu starten. Die Studie folgt auf den erfolgreichen Abschluss einer Kopfspule durch Tesla Dynamic Coils BV.
Die Studie wird AL001 mit dem vermarkteten Lithiumcarbonat vergleichen und sich auf die Pharmakokinetik von Lithium im Blut und im Gehirn bei MDD-Patienten konzentrieren. Frühere Studien an Mäusen zeigten, dass AL001 eine bessere Gehirnaufnahme erzielt, während es niedrigere Lithiumspiegel im Blut aufrechterhält, was möglicherweise die Notwendigkeit für therapeutisches Arzneimittelmonitoring (TDM) eliminiert.
AL001 zielt darauf ab, die Einschränkungen der derzeit von der FDA zugelassenen Lithiumsalze zu überwinden, die aufgrund eines engen therapeutischen Fensters regelmäßige Überwachung erfordern. Obwohl Lithium keine FDA-Zulassung zur Behandlung von MDD hat, wird es seit Jahrzehnten off-label als Ergänzungstherapie für Antidepressiva verschrieben und hat in mehreren randomisierten kontrollierten Studien Wirksamkeit gezeigt.
- Successful completion of head coil development, enabling Phase II trial progression
- Previous mouse studies demonstrated superior brain absorption with lower blood lithium levels
- Potential to eliminate need for therapeutic drug monitoring, reducing treatment burden
- Large addressable market of over 21 million Americans with MDD
- Phase II trial won't begin until Q4 2025, indicating a lengthy timeline to potential commercialization
- AL001 still lacks FDA approval for MDD treatment
- Competition from existing FDA-approved lithium treatments in the market
Insights
Alzamend's announcement of their Phase II clinical trial for AL001 represents a significant milestone in their clinical development program for MDD treatment. The trial, scheduled for Q4 2025 at Massachusetts General Hospital, follows the completion of necessary components and builds upon promising preclinical results.
The company's approach with AL001 addresses fundamental limitations of current lithium treatments. Traditional lithium therapies require continuous therapeutic drug monitoring due to their narrow therapeutic window, creating treatment barriers for patients. Preclinical data in mice demonstrates AL001's improved brain absorption with lower blood lithium levels, potentially eliminating monitoring requirements while maintaining efficacy.
For clinical context, lithium's efficacy in MDD is well-established despite lacking specific FDA approval for this indication. AL001's advancement creates a potential pathway to expand treatment options for the 21+ million Americans affected by MDD.
While this trial initiation represents forward momentum, investors should recognize this remains mid-stage clinical development. The timelines indicate results won't materialize until late 2025 or beyond, with significant regulatory hurdles remaining before commercialization. The partnership with Massachusetts General Hospital adds credibility to the trial design and execution plan, potentially strengthening future data submissions.
The advancement of AL001 to Phase II trials represents a potentially meaningful evolution in lithium-based therapies for MDD. Current lithium treatments, while effective for bipolar disorder and as off-label augmentation for depression, come with significant clinical management challenges due to their narrow therapeutic window.
The therapeutic distinction of AL001 lies in its pharmacokinetic profile - the mouse model data showing enhanced brain penetration while maintaining lower systemic concentrations addresses one of lithium's fundamental limitations. This could transform treatment paradigms by potentially eliminating the need for regular blood monitoring that currently burdens both patients and healthcare systems.
The choice to conduct head-to-head studies against lithium carbonate is methodologically sound, providing direct comparative data on both blood and brain pharmacokinetics. This approach will generate the precise evidence needed to determine if AL001 truly offers clinical advantages.
For psychiatric practice, the elimination of therapeutic drug monitoring requirements would be particularly valuable for patient populations with access to healthcare resources or those struggling with treatment adherence. However, the scheduled Q4 2025 initiation means this potential breakthrough remains distant, with several years of development still required before potential FDA consideration. The real clinical impact depends entirely on whether human trials confirm the promising preclinical pharmacokinetic profile.
- Head-to-head studies of AL001 versus a marketed lithium carbonate product will be conducted for comparisons of lithium blood and brain/brain-structure pharmacokinetics in MDD subjects
ATLANTA, March 18, 2025 (GLOBE NEWSWIRE) -- Alzamend Neuro, Inc. (Nasdaq: ALZN) (“Alzamend”), a clinical-stage biopharmaceutical company focused on developing novel products for the treatment of Alzheimer’s disease (“Alzheimer’s”), bipolar disorder (“BD”), major depressive disorder (“MDD”) and post-traumatic stress disorder (“PTSD”), today announced its plans to initiate a highly anticipated phase II clinical study of AL001 for treatment of patients with MDD in the fourth quarter of 2025. This study follows the successful completion of a head coil by Tesla Dynamic Coils BV, a key component of the clinical trial.
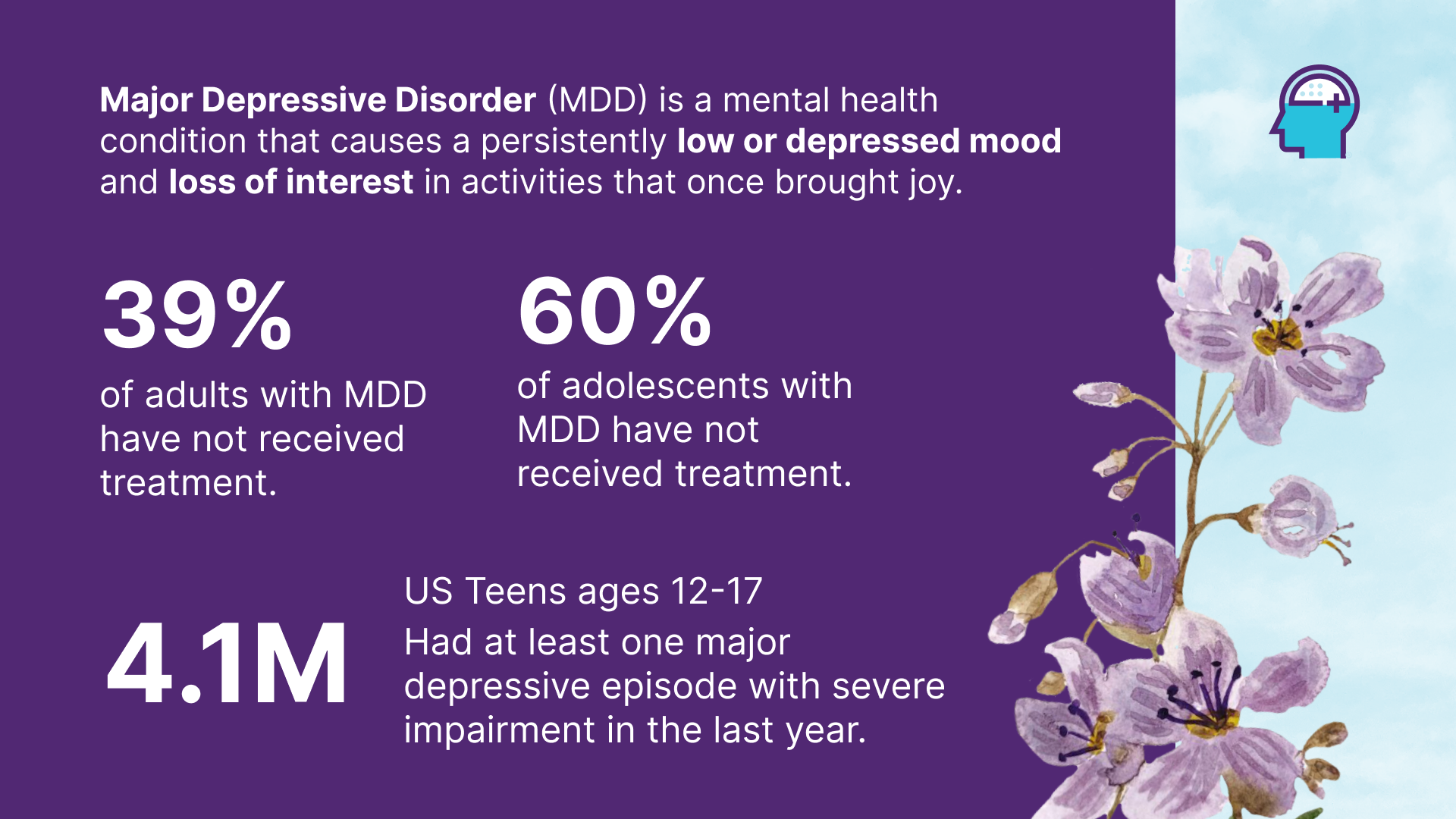
In collaboration with Massachusetts General Hospital as its contract research organization, Alzamend aims to explore the unique properties of AL001 and its effects on lithium delivery in the brain compared to marketed lithium salts. The study could illuminate the path forward in patients with MDD by demonstrating AL001’s targeted effectiveness and reduced systemic side effects. Previous studies in mice have shown that AL001 ensures better brain absorption while maintaining lower levels of lithium in the blood, paving the way for safer and more efficient treatments.

By offering a treatment that potentially eliminates the need for lithium therapeutic drug monitoring (“TDM”), AL001 could revolutionize care for vulnerable patient populations and improve treatment outcomes. Lithium, renowned for its efficacy as a first-line therapy for manic episodes and maintenance in BD, has long been underutilized due to the complexities of TDM. Current lithium salts (carbonate and citrate) approved by the U.S. Food and Drug Administration (“FDA”) are limited by a narrow therapeutic window that requires regular TDM of plasma lithium levels and blood chemistry by a clinician to mitigate adverse events. Although lithium does not have an FDA approved indication for augmentation of an antidepressant in MDD, it has been prescribed off-label for this purpose for decades. While a wide variety of medications have been used historically in this capacity, lithium is one of the few agents that has demonstrated efficacy in multiple randomized controlled trials. Although the ideal role for lithium augmentation has yet to be established, there is evidence to support the clinical practice of adding lithium to conventional antidepressants in pursuit of MDD remission.
“With AL001, we can potentially introduce a next-generation lithium treatment that offers enhanced safety, better brain targeting, and no need for TDM, promising a leap forward from the current, burdensome options,” stated Stephan Jackman, Chief Executive Officer of Alzamend. “This advancement stands to potentially enhance the lives of over 21 million Americans suffering from MDD by providing a more effective and user-friendly therapeutic option, potentially reshaping current treatment paradigms and improving patient quality of life substantially.”
About AL001
AL001 is a novel lithium-delivery system that has the potential to provide the benefits of marketed lithium salts while mitigating or avoiding currently experienced toxicities associated with lithium. Results from Alzamend’s completed Phase IIA multiple-ascending dose study of AL001 in Alzheimer’s patients and healthy subjects identified a maximum tolerated dose (“MTD”), as assessed by an independent safety review committee. This MTD is designed to be unlikely to require TDM while providing lithium at a relatively modest but effective dose. AL001 is designed to favorably distribute lithium in the brain resulting in lower exposure of other body organs and an improved safety profile compared to currently marketed lithium salts. This can serve to mitigate or obviate the disadvantageously low ceiling for toxicity of marketed lithium salts that has limited their usefulness to patients and prescribers.
About Alzamend Neuro
Alzamend Neuro is a clinical-stage biopharmaceutical company focused on developing novel products for the treatment of Alzheimer’s, BD, MDD and PTSD. Our mission is to rapidly develop and market safe and effective treatments. Our current pipeline consists of two novel therapeutic drug candidates, AL001 - a patented ionic cocrystal technology delivering lithium via a therapeutic combination of lithium, salicylate and L-proline, and ALZN002 - a patented method using a mutant-peptide sensitized cell as a cell-based therapeutic vaccine that seeks to restore the ability of a patient’s immunological system to combat Alzheimer’s by removing beta-amyloid from the brain. The latter is a second-generation active-immunity approach designed to mitigate the disadvantages of approved passive immunity marketed antibody products, particularly by reducing the required frequency and costs of dosing associated with antibody products. Both of our product candidates are licensed from the University of South Florida Research Foundation, Inc. pursuant to royalty-bearing exclusive worldwide licenses.
Forward-Looking Statements
This press release contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and include words such as “believes,” “plans,” “anticipates,” “projects,” “estimates,” “expects,” “intends,” “strategy,” “future,” “opportunity,” “may,” “will,” “should,” “could,” “potential,” or similar expressions. Statements that are not historical facts are forward-looking statements. Forward-looking statements are based on current beliefs and assumptions that are subject to risks and uncertainties. Forward-looking statements speak only as of the date they are made, and Alzamend undertakes no obligation to update any of them publicly in light of new information or future events. Actual results could differ materially from those contained in any forward-looking statement as a result of various factors. More information, including potential risk factors, that could affect Alzamend’s business and financial results are included in Alzamend’s filings with the U.S. Securities and Exchange Commission. All filings are available at www.sec.gov and on Alzamend’s website at www.Alzamend.com.
Contacts:
Email: Info@Alzamend.com or call: 1-844-722-6333
Photos accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/071d71e0-5561-409c-9f64-3d579b633cc7
https://www.globenewswire.com/NewsRoom/AttachmentNg/4b19dc00-6745-4ecd-a229-4499449361e0







