CoolSculpting® by Allergan Aesthetics Expands Body Contouring Portfolio With CoolSculpting® Elite
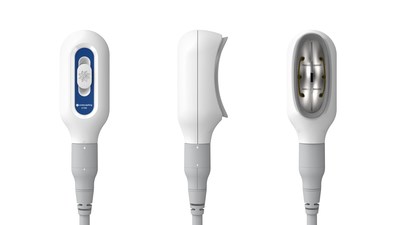
On January 26, 2021, Allergan Aesthetics, a subsidiary of AbbVie (NYSE: ABBV), launched CoolSculpting® Elite, an advanced fat reduction system designed to target and eliminate visible fat bulges in nine areas of the body. This innovative system features new C-shaped applicators that provide a better fit and comfort, along with a larger cooling area for enhanced efficacy. The product is FDA-cleared for various treatment areas and aims to address consumer concerns about excess body fat as people transition back to in-person interactions.
- Launch of CoolSculpting® Elite enhances product portfolio.
- FDA clearance for nine treatment areas increases market potential.
- New applicator designs improve treatment comfort and efficacy.
- None.
Insights
Analyzing...
IRVINE, Calif., Jan. 26, 2021 /PRNewswire/ -- Today, Allergan Aesthetics, an AbbVie company (NYSE: ABBV), announced the launch of CoolSculpting® Elite, its next generation fat reduction system with applicators designed to complement the body's natural curves. CoolSculpting® Elite harnesses proven CoolSculpting® technology to target, freeze, and eliminate treated fat cells. CoolSculpting® Elite is FDA cleared to treat visible fat bulges in nine areas of the body including the thigh, abdomen, and flank, along with bra fat, back fat, underneath the buttocks (also known as banana roll), upper arm, and the submental and submandibular areas.
"The number one aesthetic concern for consumers is excess body fat1. CoolSculpting® has been an industry-leader, creating the market for non-invasive fat reduction over 10 years ago. Now CoolSculpting® Elite builds on that heritage with a completely re-invented next-generation technology that delivers results," says Carrie Strom, Senior Vice President, AbbVie, and President, Global Allergan Aesthetics. "We understand the challenges of today's world and as people prepare to go from being online to spending more time in person, CoolSculpting® Elite can help them address their bothersome fat."
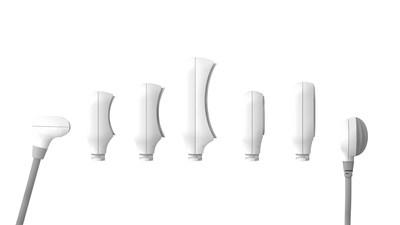
CoolSculpting® Elite will launch with a new applicator collection, which includes seven different shapes and sizes. The innovative new C-shaped applicators are designed to complement the body's natural curves for improved fit and comfort during initial tissue draw and features up to an
"CoolSculpting® is a critical part of my business and a device I've relied on to help my patients achieve their desired outcome of reducing stubborn fat." said Dr. Sabrina Fabi, Board Certified Dermatologist and Associate Research Director at Cosmetic Laser Surgery in San Diego. "The dual applicators allow us to treat two areas at the same time, safely freezing twice the fat compared to using a single CoolSculpting applicator, which helps us save on time. With the Elite applicator collection we are able to treat a wide range of body areas and personalize each patient's treatment plan."
Allergan Aesthetics™ is now taking orders for the CoolSculpting® Elite device, and the first units will ship in Q1 2021. For more information and to find a provider near you, visit http://find.coolsculpting.com/find-a-center/.
- American Society for Dermatologic Surgery (ASDS) consumer survey on cosmetic dermatologic procedures 2018 available at https://www.asds.net/skin-experts/news-room/press-releases/asds-survey-dermatologists-no-1-influencer-for-cosmetic-procedures-and-skin-care-decisions. N= 3,525 total study population. Data were obtained through a blind online survey conducted from May 1 to June 20 through the web-based Survata service.
About Allergan Aesthetics
At Allergan Aesthetics, an AbbVie company, we develop, manufacture, and market a portfolio of leading aesthetics brands and products. Our aesthetics portfolio includes facial injectables, body contouring, plastics, skin care, and more. Our goal is to consistently provide our customers with innovation, education, exceptional service, and a commitment to excellence, all with a personal touch. For more information, visit www.AllerganAesthetics.com.
About AbbVie
AbbVie's mission is to discover and deliver innovative medicines that solve serious health issues today and address the medical challenges of tomorrow. We strive to have a remarkable impact on people's lives across several key therapeutic areas: immunology, oncology, neuroscience, eye care, virology, women's health and gastroenterology, in addition to products and services across its Allergan Aesthetics portfolio. For more information about AbbVie, please visit us at www.abbvie.com. Follow @abbvie on Twitter, Facebook, Instagram, YouTube and LinkedIn.
COOLSCULPTING® AND COOLSCULPTING® ELITE IMPORTANT INFORMATION
Indications
CoolSculpting® and CoolSculpting® Elite are FDA-cleared for the treatment of visible fat bulges in the thigh, abdomen, and flank, along with bra fat, back fat, underneath the buttocks (also known as banana roll), and upper arm in patients with a Body Mass Index (BMI) of ≤ 30 and in submental and submandibular areas in patients with a BMI of ≤ 46.2. It is also FDA-cleared to affect the appearance of lax tissue with submental area treatments.
Important Safety Information
CoolSculpting® and CoolSculpting® Elite are contraindicated in patients with cryoglobulinemia, cold agglutinin disease, or paroxysmal cold hemoglobinuria.
Ask your patient about any medical conditions including recent surgery, pre-existing hernia, and any known sensitivities or allergies.
During the procedure patients may experience sensations of pulling, tugging, mild pinching, intense cold, tingling, stinging, aching, and cramping at the treatment site. These sensations subside as the area becomes numb. Following the procedure, typical side effects include temporary redness, swelling, blanching, bruising, firmness, tingling, stinging, tenderness, cramping, aching, itching, or skin sensitivity, and sensation of fullness in the back of the throat after submental or submandibular area treatment.
Rare side effects may also occur. Paradoxical hyperplasia (visibly enlarged tissue volume in the treated area) may develop 2 to 5 months after treatment and requires surgical intervention for correction.
As with any medical procedure, a consultation should be done by a licensed healthcare professional to determine if the patient is a candidate for treatment. For a complete list of Contraindications, Warnings, Precautions, and Potential Side Effects, consult the CoolSculpting® System User Manual and the CoolSculpting® Elite System User Manual. Treatment applications that deviate from the guidelines are not recommended.
COOLSCULPTING®, COOLSCULPTING® ELITE, and the Snowflake Design are trademarks of Zeltiq Aesthetics, Inc., an AbbVie Company. © 2020 AbbVie. All rights reserved.
For more information, please visit CoolSculpting.com. To report an adverse reaction, please call Allergan at 1-800-433-8871.
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/coolsculpting-by-allergan-aesthetics-expands-body-contouring-portfolio-with-coolsculpting-elite-301214607.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/coolsculpting-by-allergan-aesthetics-expands-body-contouring-portfolio-with-coolsculpting-elite-301214607.html
SOURCE AbbVie