Veeva MedTech Clinical Trials Report Signals Significant Opportunity to Improve Data Delivery and Quality
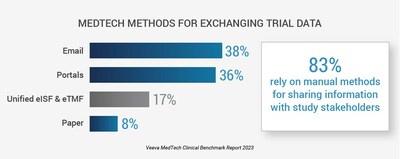
On April 19, 2023, Veeva Systems (NYSE: VEEV) released its inaugural 2023 MedTech Clinical Benchmark Report, revealing key trends in clinical trials within the medtech sector. The study found that 83% of medtech companies still use manual methods like emails and paper to share data, which hampers study efficiency and increases compliance risks. Major challenges identified include 61% of firms facing integration issues with fragmented systems. The report emphasizes a pressing need for improved collaboration and digital solutions, with 45% of respondents prioritizing a shift to digital systems in the next year. The insights from over 135 global clinical professionals highlight the urgency for modernized approaches to enhance data quality and trial execution.
- 83% of medtech companies recognize the need for improved collaboration in clinical trials.
- 45% of respondents prioritize transitioning to digital clinical systems within the next year, indicating a shift towards modernization.
- The report outlines significant opportunities for enhancing data delivery and quality in medtech studies.
- 61% of respondents face challenges due to disconnected clinical systems, leading to potential errors and inefficiencies.
- Reliance on manual data-sharing methods increases the risk of non-compliance and delays in trial execution.
Research reveals most medtech companies (
The report highlights key areas for improvement and progress made in medtech clinical research, including:
- Disconnected systems remain a crucial issue: More than half of respondents (
61% ) experience challenges with fragmented clinical systems because of cross-system integration, data management, reporting, and usability. Managing studies on siloed systems can lead to manual errors, duplicate data, and missing files. - A clear strategy is needed for post-market clinical follow-up (PMCF): There was no single method for PMCF used by most respondents, with top approaches reported as real-world evidence (
21% ), literature search (20% ), and comparison studies (20% ). Without a standard method to meet PMCF, organizations can benefit from developing an end-to-end process that spans clinical, medical, regulatory, quality, and marketing for continuous data gathering throughout the product lifecycle. - Shift to digital clinical systems accelerating this year: Nearly half (
45% ) say shifting to digital clinical systems is a top priority over the next 12 months. Establishing a digital and connected technology foundation will make it easier for study stakeholders to work together, increasing trial efficiency, accelerating data delivery, and improving the experience for sites.
"The medtech industry has a significant opportunity to modernize clinical systems and processes for faster access to trial data," said
The Veeva MedTech Clinical Benchmark study examined how organizations—ranging from emerging to large device and diagnostics companies—manage clinical processes, study site collaboration, and trial data to ensure compliance and speed. This report includes insights from more than 135 clinical medtech professionals worldwide, outlining current challenges and near-term priorities associated with clinical trial conduct. See the full report, which investigates how medtech companies are managing clinical operations, outsourcing, post-market clinical follow-up, and modernization.
About
Contact:
925-226-8821
deivis.mercado@veeva.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/veeva-medtech-clinical-trials-report-signals-significant-opportunity-to-improve-data-delivery-and-quality-301801426.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/veeva-medtech-clinical-trials-report-signals-significant-opportunity-to-improve-data-delivery-and-quality-301801426.html
SOURCE
FAQ
What does the 2023 Veeva MedTech Clinical Benchmark Report reveal?
What challenges are identified in medtech clinical trials?
How many professionals contributed to the Veeva MedTech report?
What improvements are suggested for medtech clinical trials?