Vaccinex Reports New Findings for SIGNAL-AD Phase 1b/2 Trial of Pepinemab at Clinical Trials on Alzheimer’s Disease (CTAD) Conference in Madrid, Spain
Vaccinex (VCNX) announced new findings from its SIGNAL-AD Phase 1b/2 trial of pepinemab antibody in Alzheimer's disease. The study revealed that pepinemab treatment inhibits the expression of plasma GFAP and p-tau 217 biomarkers during Mild Cognitive Impairment (MCI). The treatment showed promising trends in slowing cognitive decline in mild dementia patients with MMSE scores of 22-26. Proteomic analysis of Cerebrospinal Fluid identified several proteins typically increasing during AD progression that were inhibited by pepinemab. Additionally, using a Brain-Chip model, pepinemab demonstrated ability to inhibit or reverse damage caused by toxic alpha synuclein aggregates, suggesting potential applications in other neurodegenerative diseases.
Vaccinex (VCNX) ha annunciato nuovi risultati dal suo studio di fase 1b/2 SIGNAL-AD sull'anticorpo pepinemab nella malattia di Alzheimer. La ricerca ha rivelato che il trattamento con pepinemab inibisce l'espressione dei biomarcatori plasmatici GFAP e p-tau 217 durante il lieve deterioramento cognitivo (MCI). Il trattamento ha mostrato tendenze promettenti nel rallentare il declino cognitivo nei pazienti con demenza lieve con punteggi MMSE di 22-26. Un'analisi proteomica del fluido cerebrospinale ha identificato diverse proteine che normalmente aumentano durante la progressione della malattia di Alzheimer e che sono state inibite da pepinemab. Inoltre, utilizzando un modello Brain-Chip, pepinemab ha dimostrato la capacità di inibire o invertire i danni causati dagli aggregati tossici di alfa-sinucleina, suggerendo potenziali applicazioni in altre malattie neurodegenerative.
Vaccinex (VCNX) anunció nuevos hallazgos de su ensayo de fase 1b/2 SIGNAL-AD sobre el anticuerpo pepinemab en la enfermedad de Alzheimer. El estudio reveló que el tratamiento con pepinemab inhibe la expresión de los biomarcadores plasmáticos GFAP y p-tau 217 durante el deterioro cognitivo leve (MCI). El tratamiento mostró tendencias prometedoras en la reducción del declive cognitivo en pacientes con demencia leve con puntajes MMSE de 22-26. El análisis proteómico del líquido cerebroespinal identificó varias proteínas que normalmente aumentan durante la progresión de la enfermedad de Alzheimer y que fueron inhibidas por pepinemab. Además, utilizando un modelo Brain-Chip, pepinemab demostró la capacidad de inhibir o revertir el daño causado por agregados tóxicos de alfa-sinucleína, sugiriendo aplicaciones potenciales en otras enfermedades neurodegenerativas.
백신넥스 (VCNX)는 알츠하이머병에서 페피네맙 항체에 대한 SIGNAL-AD 1b/2상 임상시험의 새로운 발견을 발표했습니다. 연구 결과, 페피네맙 치료가 경도 인지 장애(MCI) 동안 플라스마 GFAP 및 p-tau 217 바이오마커의 발현을 억제하는 것으로 나타났습니다. 이 치료는 MMSE 점수가 22-26인 경증 치매 환자에서 인지 저하를 늦추는 데 있어 유망한 경향을 보였습니다. 단백질체 분석을 통해 알츠하이머 진행 중 일반적으로 증가하는 여러 단백질이 페피네맙에 의해 억제된 것으로 확인되었습니다. 또한, Brain-Chip 모델을 사용하여 페피네맙은 독성 알파 시누클레인 응집체에 의해 유발된 손상을 억제하거나 되돌릴 수 있는 능력을 보여주었으며, 이는 다른 신경퇴행성 질환에 대한 잠재적인 응용 가능성을 시사합니다.
Vaccinex (VCNX) a annoncé de nouvelles découvertes issues de son essai clinique de phase 1b/2 SIGNAL-AD sur l'anticorps pepinemab dans la maladie d'Alzheimer. L'étude a révélé que le traitement par pepinemab inhibe l'expression des biomarqueurs plasmatiques GFAP et p-tau 217 durant les troubles cognitifs légers (MCI). Le traitement a montré des tendances prometteuses pour ralentir le déclin cognitif chez les patients présentant une démence légère et des scores MMSE de 22 à 26. Une analyse protéomique du liquide céphalorachidien a identifié plusieurs protéines qui augmentent généralement au cours de la progression de la maladie d'Alzheimer et qui ont été inhibées par le pepinemab. De plus, en utilisant un modèle Brain-Chip, le pepinemab a démontré sa capacité à inhiber ou à inverser les dommages causés par des agrégats toxiques d'alpha-synucléine, suggérant des applications potentielles dans d'autres maladies neurodégénératives.
Vaccinex (VCNX) hat neue Erkenntnisse aus seiner SIGNAL-AD Phase 1b/2-Studie mit dem Antikörper Pepinemab bei Alzheimer veröffentlicht. Die Studie zeigte, dass die Behandlung mit Pepinemab die Expression der Plasma-Biomarker GFAP und p-tau 217 während des leichten kognitiven Abbaus (MCI) hemmt. Die Behandlung zeigte vielversprechende Tendenzen zur Verlangsamung des kognitiven Abbaus bei Patienten mit leichter Demenz mit MMSE-Werten von 22 bis 26. Eine proteomische Analyse von Hirn-Rückenmarksflüssigkeit identifizierte mehrere Proteine, die typischerweise während des Fortschreitens der Alzheimer-Krankheit zunehmen und durch Pepinemab gehemmt wurden. Darüber hinaus zeigte Pepinemab unter Verwendung eines Brain-Chip-Modells die Fähigkeit, Schäden durch toxische Alpha-Synuclein-Aggregate zu hemmen oder umzukehren, was auf potenzielle Anwendungen bei anderen neurodegenerativen Erkrankungen hindeutet.
- Pepinemab showed effectiveness in inhibiting key biomarkers (GFAP and p-tau 217) during early-stage Alzheimer's
- Treatment demonstrated promising trends in slowing cognitive decline in mild dementia patients
- No evidence of vascular damage or hemorrhage risks commonly associated with Abeta amyloid antibodies
- Potential broader application in other neurodegenerative diseases including Huntington's, Parkinson's, and Dementia with Lewy Bodies
- Company still needs to secure partnerships for continued development
- Treatment effects described as 'trends' rather than statistically significant results
Insights
The SIGNAL-AD Phase 1b/2 trial results reveal promising biomarker and cognitive treatment effects for pepinemab in early-stage Alzheimer's disease. Key findings show the drug's ability to inhibit plasma GFAP and p-tau 217 biomarkers during Mild Cognitive Impairment (MCI), suggesting potential disease-modifying effects. The drug's mechanism differs from current approved treatments by targeting astrocyte activation and inflammation rather than Abeta amyloid.
Particularly noteworthy is pepinemab's safety profile, showing no evidence of vascular damage or hemorrhage risks commonly associated with Abeta antibody treatments. The drug's ability to inhibit and reverse alpha synuclein-induced damage in Brain-Chip models suggests broader therapeutic potential across multiple neurodegenerative conditions, including Parkinson's and Dementia with Lewy Bodies.
While promising, this micro-cap company (
Company describes new biomarker and cognitive treatment effects that support continued development of pepinemab in Mild Cognitive Impairment and Mild Dementia due to Alzheimer’s disease
ROCHESTER, N.Y., Oct. 31, 2024 (GLOBE NEWSWIRE) -- Vaccinex (Nasdaq: VCNX) today provided an update on new clinical findings from its SIGNAL-AD Phase 1b/2 trial of pepinemab antibody in Alzheimer’s disease.
Vaccinex recently announced positive results of the phase 1b/2 study of its lead product, pepinemab, in early stages of Alzheimer’s disease (AD). The purpose of this report is to share additional data demonstrating stage-specific biomarker and cognitive effects starting with Mild Cognitive Impairment (MCI), the very earliest diagnostic stage of AD, and following through to progression to later stages of mild dementia. Currently approved treatments for AD are designed to reduce expression of Abeta amyloid and appear to modestly slow cognitive decline. The Vaccinex strategy is to intervene early to block astrocyte activation and associated inflammation with pepinemab so as to potentially achieve greater reduction in overall disease activity.
We report today that expression of plasma GFAP and p-tau 217 biomarkers appears to increase predominantly during MCI and that this is inhibited by pepinemab treatment. Glial Fibrillary Acidic Protein (GFAP) is a well-characterized marker of increasing astrocyte reactivity, and p-tau 217 is a peptide fragment of toxic “tau tangles” that accumulate in neurons during disease progression. In the absence of effective treatment, patients typically progress to mild dementia, which approximately corresponds to Mini-Mental State Examination (MMSE) scores of 22-26 . We report that pepinemab treatment at this stage may slow further cognitive decline as evidenced by strong treatment trends for multiple different cognitive measures.
In further support of potential treatment benefit, we performed a proteomic analysis of Cerebrospinal Fluid (CSF) from patients treated with pepinemab or placebo. The results identified several proteins that have been previously reported to increase during normal AD progression but are here shown to be inhibited by pepinemab treatment. These constitute additional disease-associated biomarkers whose inhibition, we believe, further supports the benefit of pepinemab treatment.
Finally, investors will be aware of concerns regarding damage to vascular integrity of brain tissue and the risk of inflammation and hemorrhage due to treatment with antibodies to Abeta amyloid. We have not seen evidence of such effects for treatment with pepinemab antibody. On the contrary, employing a human Brain-Chip model, we were able to demonstrate that damage caused by toxic aggregates of alpha synuclein can be inhibited or reversed by treatment with pepinemab. The results of similar analysis of damage caused by Abeta amyloid in the presence or absence of anti-Abeta antibody will be reported soon.
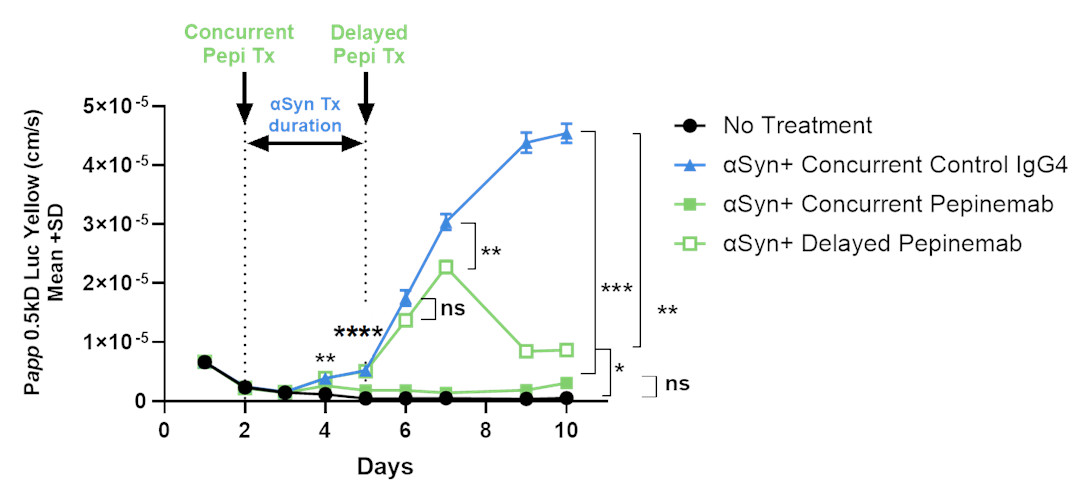
- Treatment with aSyn fibrils disrupted vascular integrity of brain chip (blue) compared to untreated control (black)
- Concurrent pepinemab treatment significantly inhibited effects of aSyn (green closed squares)
- Delayed pepinemab treatment significantly reversed effects of aSyn (green open squares)
Continued Pepinemab Development
The Company views the results of this AD study in the context of its larger previous randomized phase 2 study in Huntington’s disease (HD), which showed highly significant beneficial effects of pepinemab treatment on HD-specific biomarkers and measures of cognition. The immediate goal of the SIGNAL-AD study was to determine whether effects are consistent in another very prevalent neurodegenerative disease. Further, we report a new finding that pepinemab can inhibit or reverse the damaging effects of alpha synuclein, a key constituent of Lewy bodies that are characteristic of Neuronal Synuclein Diseases including Dementia with Lewy Bodies and Parkinson’s disease, as well as being expressed in ~
Forward Looking Statements
To the extent that statements contained in this letter are not descriptions of historical facts regarding Vaccinex, Inc. (“Vaccinex,” “we,” “us,” or “our”), they are forward-looking statements reflecting management’s current beliefs and expectations. Such statements include, but are not limited to, statements about the use and potential benefits of pepinemab treatment in patients with AD and HD; the potential and prospects for continuing late stage development of pepinemab, including as part of a prospective partnership; and other statements identified by words such as “believe,” “being,” “will,” “appear,” “expect,” “ongoing,” “potential,” “promising,” “indicate,” “suggest,” “apparent”, and similar expressions or their negatives (as well as other words and expressions referencing future events, conditions, or circumstances). Forward-looking statements involve substantial risks and uncertainties that could cause the outcome of our research and pre-clinical development programs, clinical development programs, future results, performance, or achievements to differ significantly from those expressed or implied by the forward-looking statements. Such risks and uncertainties include, among others, uncertainties inherent in the execution, cost and completion of preclinical studies and clinical trials, risks related to reliance on third parties, that interim and preliminary data may not be predictive of final results and does not ensure success in later clinical trials, uncertainties related to regulatory approval, risks related to our dependence on our lead product candidate pepinemab, the possible delisting of our common stock from Nasdaq if the Company is unable to regain and sustain compliance with the Nasdaq listing standards, and other matters that could affect our development plans or the commercial potential of our product candidates. Except as required by law, the Company assumes no obligation to update these forward-looking statements. For a further discussion of these and other factors that could cause future results to differ materially from any forward-looking statement, see the section titled “Risk Factors” in our periodic reports filed with the Securities and Exchange Commission and the other risks and uncertainties described in the Company’s annual year-end Form 10-K and subsequent filings with the SEC.
Investor Contact
Elizabeth Evans, PhD
Chief Operating Officer, Vaccinex, Inc.
(585) 271-2700
eevans@vaccinex.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/775d3ef7-a1e1-41c0-9f61-c7f4ddec61b5








