Vivani Medical Announces Positive Preclinical Weight Loss Data for NPM-139 Semaglutide Implant, with Potential for Once-Yearly Dosing
Vivani Medical (NASDAQ: VANI) has announced promising preclinical data for NPM-139, its subdermal semaglutide implant for chronic weight management. The implant, utilizing their proprietary NanoPortal™ technology, demonstrated nearly 20% placebo-adjusted weight loss from a single administration in healthy rats over a 91-day treatment period.
The technology successfully delivers semaglutide (active ingredient in Ozempic®/Wegovy®) with potential for once or twice-yearly dosing. NPM-139 has shown therapeutic semaglutide exposure levels over six months in healthy rats, with in vitro stability measurements indicating potential for once-yearly administration.
The company's ongoing LIBERATE-1 clinical study for NPM-115 (exenatide implant) is progressing, with top-line data expected by mid-2025. This study will provide critical information for both NPM-115 and NPM-139 development, representing the first human application of NanoPortal technology.
Vivani Medical (NASDAQ: VANI) ha annunciato dati preclinici promettenti per NPM-139, il suo impianto sottocutaneo di semaglutide per la gestione cronica del peso. L'impianto, che utilizza la loro tecnologia proprietaria NanoPortal™, ha dimostrato una perdita di peso aggiustata per il placebo di quasi il 20% da una singola somministrazione in ratti sani durante un periodo di trattamento di 91 giorni.
La tecnologia riesce a somministrare semaglutide (principio attivo di Ozempic®/Wegovy®) con potenziale per somministrazioni una o due volte all'anno. NPM-139 ha mostrato livelli terapeutici di esposizione a semaglutide per sei mesi in ratti sani, con misurazioni di stabilità in vitro che indicano un potenziale per una somministrazione annuale.
Lo studio clinico in corso LIBERATE-1 per NPM-115 (impianto di exenatide) sta progredendo, con risultati preliminari attesi entro metà del 2025. Questo studio fornirà informazioni critiche per lo sviluppo di NPM-115 e NPM-139, rappresentando la prima applicazione umana della tecnologia NanoPortal.
Vivani Medical (NASDAQ: VANI) ha anunciado datos preclínicos prometedores para NPM-139, su implante subdérmico de semaglutida para el manejo crónico del peso. El implante, que utiliza su tecnología patentada NanoPortal™, demostró una pérdida de peso ajustada por placebo de casi el 20% tras una única administración en ratas sanas durante un período de tratamiento de 91 días.
La tecnología logra administrar semaglutida (ingrediente activo en Ozempic®/Wegovy®) con potencial para dosis anuales o semestrales. NPM-139 ha mostrado niveles terapéuticos de exposición a semaglutida durante seis meses en ratas sanas, con mediciones de estabilidad in vitro que indican un potencial para administración anual.
El estudio clínico en curso LIBERATE-1 para NPM-115 (implante de exenatida) está avanzando, con datos preliminares esperados para mediados de 2025. Este estudio proporcionará información crítica para el desarrollo de NPM-115 y NPM-139, representando la primera aplicación humana de la tecnología NanoPortal.
비바니 메디컬 (NASDAQ: VANI)은 만성 체중 관리를 위한 NPM-139의 유망한 전임상 데이터를 발표했습니다. 이 임플란트는 자사의 독자적인 NanoPortal™ 기술를 활용하여, 91일 치료 기간 동안 건강한 쥐에서 단일 투여로 약 20%의 위약 조정 체중 감소를 보여주었습니다.
이 기술은 세마글루타이드(오젬픽®/웨고비®의 활성 성분)를 성공적으로 전달하며, 연 1회 또는 2회 투여의 가능성을 가지고 있습니다. NPM-139는 건강한 쥐에서 6개월 동안 세마글루타이드에 대한 치료적 노출 수준을 보여주었으며, 인 비트로 안정성 측정 결과 연 1회 투여의 가능성을 나타냈습니다.
현재 진행 중인 LIBERATE-1 임상 연구는 NPM-115(엑세나타이드 임플란트)에 대한 데이터가 2025년 중반에 예상되고 있으며, 이 연구는 NPM-115와 NPM-139 개발을 위한 중요한 정보를 제공할 것입니다. 이는 NanoPortal 기술의 첫 번째 인간 적용 사례를 나타냅니다.
Vivani Medical (NASDAQ: VANI) a annoncé des données précliniques prometteuses pour NPM-139, son implant sous-cutané de sémaglutide pour la gestion chronique du poids. L'implant, utilisant leur technologie propriétaire NanoPortal™, a démontré une perte de poids ajustée au placebo de près de 20 % après une seule administration chez des rats sains sur une période de traitement de 91 jours.
La technologie permet de délivrer de la sémaglutide (ingrédient actif de l'Ozempic®/Wegovy®) avec un potentiel de dosage une ou deux fois par an. NPM-139 a montré des niveaux d'exposition thérapeutique à la sémaglutide pendant six mois chez des rats sains, avec des mesures de stabilité in vitro indiquant un potentiel pour une administration annuelle.
L'étude clinique en cours LIBERATE-1 pour NPM-115 (implant d'exénatide) progresse, avec des données préliminaires attendues d'ici mi-2025. Cette étude fournira des informations cruciales pour le développement de NPM-115 et NPM-139, représentant la première application humaine de la technologie NanoPortal.
Vivani Medical (NASDAQ: VANI) hat vielversprechende präklinische Daten für NPM-139, sein subdermales Semaglutid-Implantat zur chronischen Gewichtsregulation, bekannt gegeben. Das Implantat, das ihre proprietäre NanoPortal™-Technologie nutzt, zeigte über einen Behandlungszeitraum von 91 Tagen bei gesunden Ratten einen nahezu 20%igen gewichtsreduzierenden Effekt im Vergleich zur Placebo-Gruppe nach einer einzigen Verabreichung.
Die Technologie ermöglicht die erfolgreiche Abgabe von Semaglutid (Wirkstoff in Ozempic®/Wegovy®) mit dem Potenzial für einmal oder zweimal jährliche Dosen. NPM-139 hat über einen Zeitraum von sechs Monaten therapeutische Semaglutid-Spiegel bei gesunden Ratten gezeigt, wobei In-vitro-Stabilitätsmessungen auf ein Potenzial für eine jährliche Verabreichung hinweisen.
Die laufende klinische Studie LIBERATE-1 für NPM-115 (Exenatide-Implantat) schreitet voran, wobei erste Ergebnisse bis Mitte 2025 erwartet werden. Diese Studie wird entscheidende Informationen für die Entwicklung von NPM-115 und NPM-139 liefern und stellt die erste menschliche Anwendung der NanoPortal-Technologie dar.
- Preclinical data shows 20% weight loss vs placebo over 91 days
- Demonstrated 6-month therapeutic exposure levels in rats
- Potential for once-yearly dosing based on stability data
- Addresses $25B semaglutide market opportunity
- Successfully enrolled and implanted first subjects in LIBERATE-1 study
- Still in preclinical stage for NPM-139
- No human data available yet for the NanoPortal technology
- Clinical results not expected until mid-2025
Insights
Vivani's preclinical data for its semaglutide implant represents a potentially significant advancement in the white-hot GLP-1 market. With nearly
The market opportunity is substantial. With over half of current GLP-1 patients regularly missing doses according to real-world data, a long-acting implant that guarantees adherence could capture significant market share and potentially expand the overall patient population. Current GLP-1 products require weekly or daily injections, creating a major convenience gap that NPM-139 could fill.
While still in preclinical stage, these results provide compelling evidence for the viability of Vivani's NanoPortal™ platform technology. The translation to humans remains to be proven, but the parallel advancement of their NPM-115 exenatide implant into first-in-human studies provides some validation of their technological approach and regulatory pathway.
For a company with just a
The NanoPortal™ implant technology showcased in Vivani's preclinical results solves fundamental challenges in GLP-1 therapy delivery. The subdermal implant design achieves two critical objectives that current injection methods can't: guaranteed medication adherence and smoother pharmacokinetics.
The technical achievement here is remarkable. Maintaining stable semaglutide release over 91 days while demonstrating in vitro stability exceeding one year requires sophisticated drug stabilization and controlled-release engineering. This suggests Vivani has overcome significant technical hurdles in protein stability and controlled diffusion mechanisms.
The smooth, non-fluctuating release profile could potentially reduce the GI side effects that cause many patients to discontinue traditional GLP-1 therapies. Peak-and-trough dosing from injections often triggers nausea and other adverse events at plasma concentration peaks. A flat pharmacokinetic profile would minimize these spikes.
Implementation requires minimally invasive procedures for implantation and removal, likely performed in outpatient settings. The study suggests the implant is miniaturized enough for practical clinical use. The platform versatility is equally impressive - demonstrating efficacy with both exenatide (NPM-115) and semaglutide (NPM-139) indicates the technology can be adapted to multiple peptide therapeutics.
While the technology shows promise, human studies will need to confirm biocompatibility, implant tolerability, and verify that the in vivo release kinetics translate from animal models to patients. The upcoming LIBERATE-1 data will provide crucial validation of the platform's clinical performance.
NanoPortal™ technology successfully delivers semaglutide, the active ingredient in Ozempic®/Wegovy®, in a preclinical study with NPM-139 (semaglutide implant)
NPM-139 treatment resulted in nearly
NPM-139 is a miniature, subdermal implant in development for chronic weight management designed to guarantee medication adherence and potentially improve treatment tolerability by providing smooth and steady delivery of GLP-1 therapy
ALAMEDA, Calif., March 26, 2025 (GLOBE NEWSWIRE) -- Vivani Medical, Inc. (NASDAQ: VANI) (“Vivani” or the “Company”), a clinical-stage biopharmaceutical company developing miniature, ultra long-acting drug implants, today announced promising preclinical data for NPM-139, its subdermal semaglutide implant under development for chronic weight management in obese and overweight individuals. These results reinforce the company’s commitment to addressing chronic weight management and other chronic diseases by leveraging its proprietary NanoPortal™ implant technology which is designed to enable smooth and steady delivery of therapeutic molecules including GLP-1 therapy. This development marks a significant advancement in improving medication adherence and patient convenience, addressing a critical gap in the treatment of chronic diseases including obesity and type 2 diabetes.
“Products containing semaglutide generated
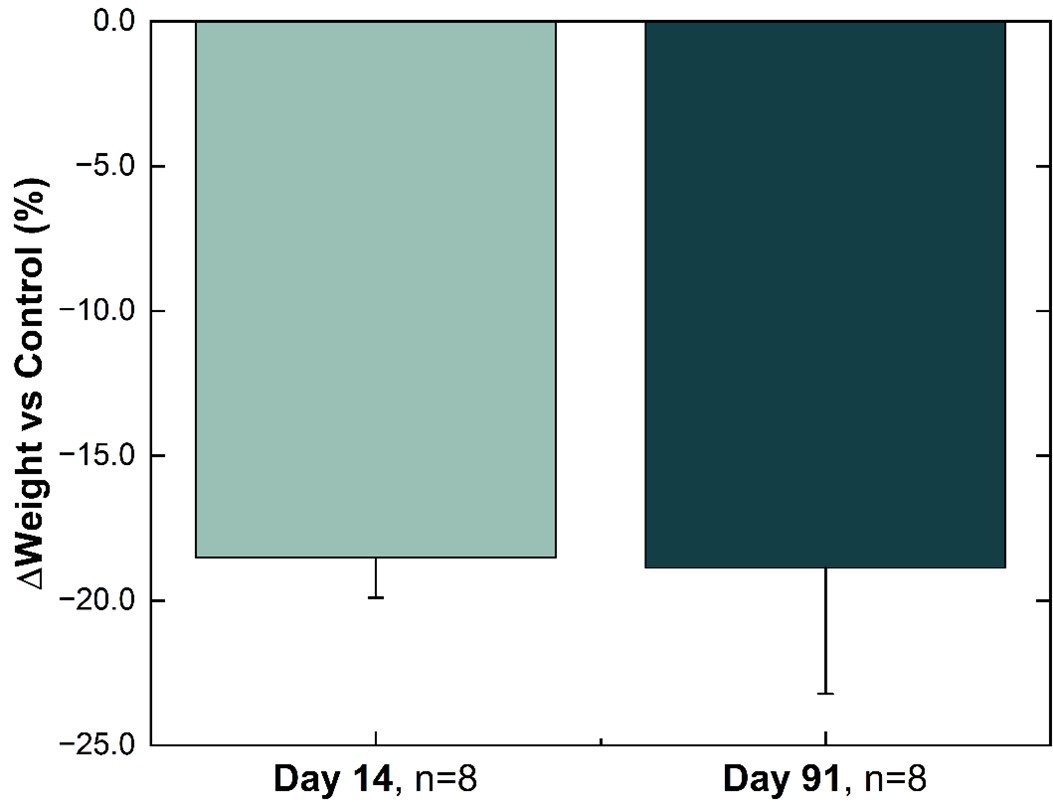
Weight difference versus control group in healthy Sprague-Dawley Rats. % weight change from baseline for NPM-139 (semaglutide) corrected to control (sham implant). Values are mean ± SE.
In an ongoing study in healthy rats, a single administration of the semaglutide implant NPM-139 resulted in body weights that were nearly
The ongoing NPM-115 clinical study, LIBERATE-1, for which the first successful implantation was recently announced, remains on track to produce top-line data by mid-2025. While LIBERATE-1 will primarily inform continued development of NPM-115, LIBERATE-1 will also provide critical information to support the development of NPM-139 and other pipeline programs since it represents the first human application of the NanoPortal technology.
About LIBERATE-1
LIBERATE-1 is a Phase 1, first-in-human study of a miniature, ultra long-acting GLP-1 (exenatide) implant, NPM-115, to investigate the safety, tolerability, and full pharmacokinetic profile in obese or overweight subjects. The trial has enrolled participants who are intended to be titrated on weekly semaglutide injections for 8 weeks (0.25 mg/week for 4 weeks followed by 0.5 mg/week for 4 weeks) before being randomized to receive a single administration of Vivani’s exenatide implant (NPM-115, n=8), weekly exenatide injections (Bydureon BCise®, n=8), or weekly 1 mg semaglutide injections (Wegovy®, n=8) for a 9-week treatment duration. Changes in weight will be measured. The study is currently on-going at two study centers in Australia and is fully enrolled. Top-line data from the study is anticipated to be available in mid-2025.
Vivani has successfully utilized research and development rebates from the Australian government for relevant 2024 expenses to defray a portion of the costs from this clinical trial and anticipates being able to utilize similar rebates going forward. Since clinical studies conducted in Australia comply with the International Conference on Harmonization guidelines, data generated in Australia generally are acceptable to the U.S. Food and Drug Administration and other regulatory authorities. Vivani anticipates use of relevant clinical data generated in Australia to support regulatory submissions in other geographies including the United States. Additional guidance regarding future regulatory submissions will be provided as new information becomes available.
Bydureon BCise® is a registered trademark under license by AstraZeneca.
Ozempic® and Wegovy® are registered trademarks of Novo Nordisk A/S.
About Vivani Medical, Inc.
Leveraging its proprietary NanoPortal™ platform, Vivani develops biopharmaceutical implants designed to deliver drug molecules steadily over extended periods of time with the goal of guaranteeing adherence, and potentially to improve patient tolerance to their medication. Vivani’s lead program, NPM-115, is a six-month, subdermal, GLP-1 (exenatide) implant under development for chronic weight management in obese or overweight individuals. Vivani’s emerging pipeline includes NPM-139 (semaglutide implant) which is also under development for chronic weight management. The semaglutide implant is being initially developed as a twice-yearly implant but it has the added potential benefit of once-yearly administration. NPM-119 refers to the Company’s six-month, subdermal, GLP-1 (exenatide) implant under development for the treatment of type 2 diabetes. These NanoPortal implants are designed to provide patients with the opportunity to realize the full potential benefit of their medication by avoiding the challenges associated with the daily or weekly administration of orals and injectables. Medication non-adherence occurs when patients do not take their medication as prescribed. This affects an alarming number of patients, approximately
Forward-Looking Statements
This press release contains certain “forward-looking statements” within the meaning of the “safe harbor” provisions of the US Private Securities Litigation Reform Act of 1995. Forward-looking statements can be identified by words such as: “target,” “believe,” “expect,” “will,” “may,” “anticipate,” “estimate,” “would,” “positioned,” “future,” and other similar expressions that in this press release, including statements regarding Vivani’s business, products in development, including the therapeutic potential thereof, the planned development therefor, the completion of the LIBERATE-1 trial and reporting of trial results, Vivani’s emerging development plans for NPM-115, NPM-139, NPM-119, or Vivani’s plans with respect to its wholly owned subsidiary, Cortigent Inc., and Vivani’s technology, strategy, cash position and financial runway. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on Vivani’s current beliefs, expectations, and assumptions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict and many of which are outside of Vivani’s control. Actual results and outcomes may differ materially from those indicated in the forward-looking statements. Therefore, you should not rely on any of these forward-looking statements. Important factors that could cause actual results and outcomes to differ materially from those indicated in the forward-looking statements include, among others, risks related to the development and commercialization of Vivani’s products, including NPM-115, NPM-139, and NPM-119; delays and changes in the development of Vivani’s products, including as a result of applicable laws, regulations and guidelines, potential delays in submitting and receiving regulatory clearance or approval to conduct Vivani’s development activities; risks related to the initiation, enrollment and conduct of Vivani’s planned clinical trials and the results therefrom; Vivani’s history of losses and Vivani’s ability to access additional capital or otherwise fund Vivani’s business; market conditions and the ability of Cortigent to complete its intended spin-off from the Company. There may be additional risks that the Company considers immaterial, or which are unknown. A further list and description of risks and uncertainties can be found in the Company’s most recent Annual Report on Form 10-K filed with the U.S. Securities and Exchange Commission filed on March 26, 2024, as updated by the Company’s subsequent Quarterly Reports on Form 10-Q. Any forward-looking statement made by Vivani in this press release is based only on information currently available to the Company and speaks only as of the date on which it is made. The Company undertakes no obligation to publicly update any forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of added information, future developments or otherwise, except as required by law.
Company Contact:
Donald Dwyer
Chief Business Officer
info@vivani.com
(415) 506-8462
Investor Relations Contact:
Jami Taylor
Investor Relations Advisor
investors@vivani.com
(415) 506-8462
Media Contact:
Sean Leous
ICR Healthcare
Sean.Leous@ICRHealthcare.com
(646) 866-4012
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/65d3448c-2cdb-4b4c-8b8d-370fa2aa308d







