Vivani Medical Announces Positive Preclinical Liver Fat Results with Miniature, Ultra Long-Acting GLP-1 Implant
Vivani’s GLP-1 (exenatide) implant produced sham-implant adjusted liver fat reduction of
Vivani previously announced sham-implant adjusted preclinical weight loss of
Clinical development of Vivani’s exenatide implant in overweight and obese patients as part of the Company’s NPM-115 program remains on track to commence in the fourth quarter of 2024
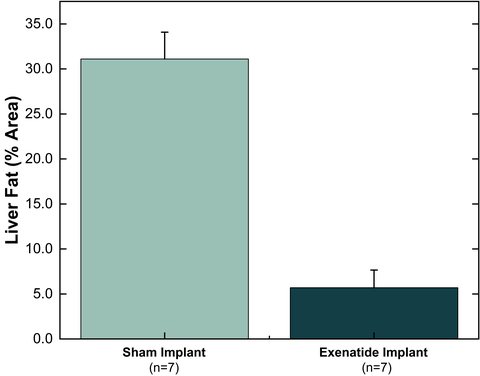
Liver fat % area for exenatide implant vs sham implant 12 weeks after a single administration. Liver fat % area is calculated using Oil Red O (ORO) staining. Values are mean ± SE. (Graphic: Business Wire)
“The reduction in liver fat observed preclinically with our miniature, subdermal exenatide implant, provides further support that the NanoPortal™ implant technology continues to hold great potential as a highly differentiated treatment option for the treatment of obesity and chronic weight management as well as related metabolic disorders,” said Adam Mendelsohn, Ph.D., Vivani President and Chief Executive Officer. “While the currently marketed GLP-1 based drugs have made profound improvements in the treatment of chronic diseases, including obesity and chronic weight management, significant barriers to optimal patient outcomes persist due to medication non-adherence and poor tolerability. We remain committed to the development of our portfolio of innovative, miniature, GLP-1 implant candidates and their potential to improve real-world patient outcomes by improving medication adherence. In addition, we believe that our implant’s minimally fluctuating release profile combined with avoiding inevitable drug fluctuations that result from poor adherence may have the potential to improve treatment tolerability, a primary complaint associated with currently available GLP-1 treatment options. Our first clinical study, LIBERATE-1™, is anticipated to start in the fourth quarter of 2024 with results in 2025.”
Liver Fat Reduction in High Fat Diet-induced Obese Mice
These liver fat data are consistent with published results from similar investigations with semaglutide, the active pharmaceutical ingredient in Ozempic and Wegovy.
Previously reported results from this study in high-fat diet-induced obese mice demonstrated that Vivani’s exenatide implant generated weight loss of approximately
Weight Loss in High Fat Diet-induced Obese Mice
NPM-115 Program
In July 2024, Vivani provided an update on the clinical development plans for its obesity program, NPM-115, which will evaluate the Company’s miniature, long-acting exenatide implant in obese and overweight patients. In support of the recent strategic shift to prioritize the development of its obesity and chronic weight management portfolio, the Company announced revised plans to evaluate its GLP-1 implant as part of the NPM-115 program in individuals who are obese or overweight in the Company’s first-in-human study, LIBERATE-1. This study will enroll participants who will be titrated on weekly semaglutide (Wegovy) for eight weeks before being randomized to receive a single exenatide implant, weekly exenatide injections (Bydureon BCise®), or weekly semaglutide injections for a nine-week treatment duration. The Company expects the study to be initiated in the fourth quarter of 2024 in
Ozempic® and Wegovy® are a registered trademarks of Novo Nordisk A/S. Bydureon BCise® is a registered trademark of the AstraZeneca group of companies.
About Vivani Medical, Inc.
Leveraging its proprietary NanoPortal™ platform, Vivani develops biopharmaceutical implants designed to deliver drug molecules steadily over extended periods of time with the goal of guaranteeing adherence, and potentially to improve tolerance to their medication. Vivani’s lead program, NPM-115, will evaluate a miniature, six-month, subdermal, GLP-1 (exenatide) implant for chronic weight management in obese or overweight individuals. Vivani’s emerging pipeline also includes NPM-139 (semaglutide implant) which is also under development for chronic weight management in obese and overweight patients. NPM-139 has the added potential benefit of once-yearly administration. NPM-119 refers to the Company’s clinical program which will evaluate its six-month, subdermal exenatide implant for the treatment of type 2 diabetes. These NanoPortal implants are designed to provide individuals with the opportunity to realize the full potential benefit of their medication by avoiding the challenges associated with the daily or weekly administration of orals and injectables. Medication non-adherence affects an alarming number of patients, approximately
Forward-Looking Statements
This press release contains certain “forward-looking statements” within the meaning of the “safe harbor” provisions of the US Private Securities Litigation Reform Act of 1995. Forward-looking statements can be identified by words such as: “target,” “believe,” “expect,” “will,” “may,” “anticipate,” “estimate,” “would,” “positioned,” “future,” and other similar expressions that in this press release, including statements regarding Vivani’s business, products in development, including the therapeutic potential thereof, the planned development therefor, the initiation of the LIBERATE-1 trial and reporting of trial results, Vivani’s emerging development plans for NPM-115, NPM-139, and NPM-119. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on Vivani’s current beliefs, expectations, and assumptions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict and many of which are outside of Vivani’s control. Actual results and outcomes may differ materially from those indicated in the forward-looking statements. Therefore, you should not rely on any of these forward-looking statements. Important factors that could cause actual results and outcomes to differ materially from those indicated in the forward-looking statements include, among others, risks related to the development and commercialization of Vivani’s products, including NPM-115, NPM-139, and NPM-119; delays and changes in the development of Vivani’s products, including as a result of applicable laws, regulations and guidelines, potential delays in submitting and receiving regulatory clearance or approval to conduct Vivani’s development activities, including Vivani’s ability to commence clinical development of NPM-115; risks related to the initiation, enrollment and conduct of Vivani’s planned clinical trials and the results therefrom; Vivani’s history of losses and Vivani’s ability to access additional capital or otherwise fund Vivani’s business. There may be additional risks that the Company considers immaterial, or which are unknown. A further list and description of risks and uncertainties can be found in the Company’s most recent Annual Report on Form 10-K filed with the
View source version on businesswire.com: https://www.businesswire.com/news/home/20240904740218/en/
Company Contact:
Donald Dwyer
Chief Business Officer
info@vivani.com
(415) 506-8462
Investor Relations Contact:
Jami Taylor
Investor Relations Advisor
investors@vivani.com
(415) 506-8462
Media Contact:
Sean Leous
ICR Westwicke
sean.leous@westwicke.com
(646) 866-4012
Source: Vivani Medical, Inc.







