Theralase(R) Successfully Destroys Lung Cancer
Theralase Technologies announced that its lead compound, Ruvidar, combined with transferrin to form Rutherrin, has shown preclinical effectiveness in destroying Non-Small Cell Lung Cancer (NSCLC). Experiments using a Lewis Lung Cancer model demonstrated that Rutherrin stays longer in tumor tissues than in normal lung tissues, leading to enhanced selectivity. All mice treated with x-ray activated Rutherrin survived, showing up to a 4-fold slower tumor progression compared to those treated with radiation alone. Theralase plans to start a Phase Ia clinical study for GBM and NSCLC in Q4 2024, with possible expansions into other cancers like pancreatic, prostate, kidney, and colorectal cancers, pending successful GLP toxicology results and capitalization.
- Effective preclinical results of Rutherrin in destroying NSCLC.
- Rutherrin shows enhanced selectivity by staying longer in tumor tissues.
- Mice treated with Rutherrin and x-ray survived with significantly slower tumor progression.
- Potential for Rutherrin to treat other cancers: pancreatic, prostate, kidney, and colorectal.
- Phase Ia clinical trials planned for Q4 2024 for GBM and NSCLC.
- Clinical effectiveness yet to be proven in humans.
- Pending successful completion of GLP toxicology program before clinical trials can commence.
- Potential financial risk due to pending capitalization for further research and trials.
TORONTO, ON / ACCESSWIRE / June 6, 2024 / Theralase® Technologies Inc. ("Theralase®" or the "Company") (TSXV:TLT)(OTCQB:TLTFF) is a clinical stage pharmaceutical company that is dedicated to the research and development of light and/or radiation activated small molecules for the safe and effective destruction of various cancers, bacteria and viruses, is pleased to announce that it's lead compound, RuvidarTM, combined with transferrin to form the compound Rutherrin®, has been proven effective preclinically in the destruction of Non-Small Cell Lung Cancer ("NSCLC").
Theralase® recently completed experiments in NSCLC, using a Lewis Lung Cancer ("LLC1") orthotopic model. In this model, mouse lungs are subjected to lung cancer cells, which induces these mice to develop very aggressive, fast growing and metastatic lung tumours.
As shown in Figure 1, lung tumours retained Rutherrin® longer than normal lung tissues (p> 0.01), leading to a substantially improved selectivity of Rutherrin® to target lung cancer.
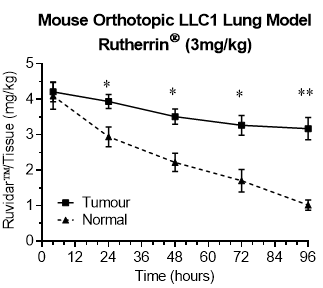
Figure 1: Rutherrin® concentration in normal and tumour lung after single 3mg/kg Intra Venous ("IV') injection
Theralase® has demonstrated that all animals treated with x-ray activated Rutherrin® are currently alive; unfortunately, this is not the case for mice that received x-ray treatment only.
In addition, the mice treated with x-ray activated Rutherrin® have demonstrated up to a 4-fold slower tumour progression, based on the Magnetic Resonance Imaging ("MRI") assessment of tumour volumes.
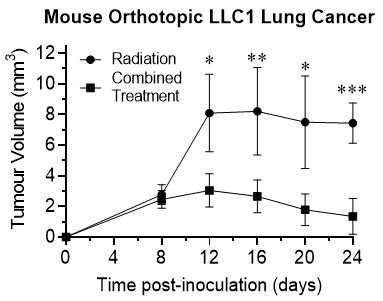
Figure 2: Tumour volume analysis in mice after tumour inoculation and treatment with either radiation only or combined treatment of Rutherrin ® and radiation treatment
As shown in Figure 2, there is a significant delay in tumour progression in mice treated with x-ray activated Rutherrin® versus with radiation alone (p> 0.001). In fact, in mice treated with x-ray activated Rutherrin®, the tumour is notably regressing / being destroyed over time.
Dr. Arkady Mandel, M.D., Ph.D., D.Sc., Chief Scientific Officer of Theralase® stated, "Between 30 to
Roger DuMoulin-White, B.Sc., P.Eng., Pro.Dir., President and Chief Executive Officer of Theralase® stated "Pending the successful completion of the Good Laboratory Practice ("GLP") toxicology program, Theralase® intends to commence a Phase Ia dose escalating clinical study in 4Q2024 for patients diagnosed with GBM and NSCLC, to determine the appropriate clinical dose of Rutherrin® to be administered from a safety perspective; including: toxicity and tumour localization. This research may expand to include: pancreatic cancer, prostate cancer, kidney cancer and colorectal cancer, based on capitalization. Following successful completion of Phase Ia, a Phase Ib study will be commenced to determine patient efficacy, using x-ray activated Rutherrin®. Pending the successful completion of the Phase Ia/Ib clinical study, Theralase® plans to commence a Phase II clinical study in Canada and the United States focused on enrolling and treating patients diagnosed with GBM and NSCLC, which as previously mention may expand to include patients diagnosed with pancreatic cancer, prostate cancer, kidney cancer and colorectal cancer, subject to capitalization.
About Lung Cancer:
Lung cancer is the leading cause of cancer mortality in men aged ≥40 years and women aged ≥60 years, causing more deaths than any other dominant cancer of men or women. The largest demographic (80 to
About Theralase® Technologies Inc.:
Theralase® is a clinical stage pharmaceutical company dedicated to the research and development of light and/or radiation activated small molecule compounds, their associated drug formulations and the light systems that activate them, with a primary objective of efficacy and a secondary objective of safety in the destruction of various cancers, bacteria and viruses.
Additional information is available at www.theralase.com and www.sedar.com
Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
Forward Looking Statements:
This news release contains "forward-looking statements" within the meaning of applicable Canadian securities laws. Such statements include; but are not limited to statements regarding the Company's proposed development plans with respect to Photo Dynamic Compounds and their drug formulations. Forward looking statements may be identified by the use of the words "may, "should", "will", "anticipates", "believes", "plans", "expects", "estimate", "potential for" and similar expressions; including, statements related to the current expectations of Company's management for future research, development and commercialization of the Company's Photo Dynamic Compounds and their drug formulations, preclinical research, clinical studies and regulatory approvals.
These statements involve significant risks, uncertainties and assumptions; including, the ability of the Company to fund and secure the regulatory approvals to successfully complete a Phase II NMIBC clinical study in a timely fashion and implement its development plans. Other risks include: the ability of the Company to successfully commercialize its drug formulations, the risk that access to sufficient capital to fund the Company's operations may not be available on terms that are commercially favorable to the Company or may not be available at all, the risk that the Company's drug formulations may not be effective against the diseases tested in its clinical studies, the risk that the Company's fails to comply with the term of license agreements with third parties and as a result loses the right to use key intellectual property in its business, the Company's ability to protect its intellectual property, the timing and success of submission, acceptance and approval of regulatory filings. Many of these factors that will determine actual results are beyond the Company's ability to control or predict.
Readers should not unduly rely on these forward-looking statements, which are not a guarantee of future performance. There can be no assurance that forward-looking statements will prove to be accurate as such forward-looking statements involve known and unknown risks, uncertainties and other factors which may cause actual results or future events to differ materially from the forward-looking statements.
Although the forward-looking statements contained in the press release are based upon what management currently believes to be reasonable assumptions, the Company cannot assure prospective investors that actual results, performance or achievements will be consistent with these forward-looking statements.
All forward-looking statements are made as of the date hereof and are subject to change. Except as required by law, the Company assumes no obligation to update such statements.
For investor information on the Company, please feel to reach out Investor Inquiries - Theralase Technologies.
For More Information:
1.866.THE.LASE (843-5273)
416.699.LASE (5273)
www.theralase.com
Kristina Hachey, CPA
Chief Financial Officer
X 224
khachey@theralase.com
SOURCE: Theralase Technologies Inc.
View the original press release on accesswire.com







