Stryker Launches Q Guidance System with Spine Guidance Software
Stryker announced the launch of its Q Guidance System, the first spine navigation software cleared by the FDA for pediatric patients aged 13 and older. This advanced system integrates a redesigned camera and Spine Guidance Software to enhance surgical planning and navigation for spine applications. The system boasts several features, including a proprietary 4th generation camera and improved image processing capabilities. Stryker aims to expand this technology across various surgical specialties in the future.
- Q Guidance System is the first spine navigation software cleared by FDA for pediatric patients aged 13 and older.
- Features advanced optical tracking and a proprietary 4th generation FP8000 camera.
- Enhanced surgical planning and navigation capabilities with Spine Guidance Software.
- None.
First spine navigation software to receive clearance from the FDA for use with pediatric patients aged 13 and older
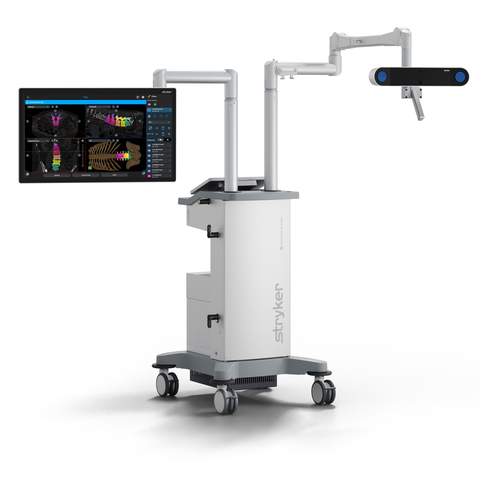
The Q Guidance System combines new optical tracking options provided by a redesigned, state-of-the-art camera with sophisticated algorithms of the newly launched
“The Q Guidance System offers cutting-edge tracking options with its 4th generation FP8000 camera, and
Q Guidance offers surgeons numerous benefits, including:
- A proprietary camera: The Q Guidance System features a 4th generation FP8000 camera that offers unmatched speeds and the flexibility of multiple optical tracking methods, including full-spectrum active/passive hybrid optical tracking.1 It is the only guidance system with proprietary active technology and a non-invasive patient tracker, SpineMask.
-
Image processing:
Spine Guidance Software features completely redesigned applications, semi-automatic and automatic processing features, gesture recognition, and broad compatibility with various types of image sets. -
Spine Guidance Software :Spine Guidance Software is designed to help surgeons optimize their workflow, minimize their time in the OR and address complex clinical decisions and techniques intraoperatively. Its computational power is designed to support our spine product and software roadmap. It is our intention to develop and release new technologies within the Q ecosystem.
“In my experience,
Stryker will be featuring the Q Guidance System and offering demonstrations at several upcoming meetings, including:
-
Society for Minimally Invasive Spine Surgery (SMISS) 2022 Annual Forum inLas Vegas , Sept. 29–Oct. 1, 2022 (booth #206, #208, #210) -
Congress of Neurological Surgeons (CNS) 2022 Annual Meeting inSan Francisco , Oct. 8–12, 2022 (booth #419) -
North American Spine Society (NASS) Annual Meeting inChicago , Oct. 12–15, 2022 (booth #4211)
About Stryker
Stryker is one of the world’s leading medical technology companies and, together with its customers, is driven to make healthcare better. The company offers innovative products and services in Medical and Surgical, Neurotechnology, and Orthopaedics and Spine that help improve patient and hospital outcomes. Alongside its customers around the world, Stryker impacts more than 100 million patients annually. More information is available at www.stryker.com.
Dr.
References:
- Stryker data on file
Content ID: QGS-PR-2_33777
View source version on businesswire.com: https://www.businesswire.com/news/home/20220927005511/en/
Media contact
562.304.0301
asampson@sampsonprgroup.com
Source: Stryker
FAQ
What is the Q Guidance System from Stryker?
When was the Q Guidance System launched by Stryker?
What are the features of the Q Guidance System?
Which patients can use the Q Guidance System?







