Stryker Announces Launch of Pangea Plating System
Stryker (NYSE: SYK) has launched its Pangea Plating System, which received FDA clearance in late 2023. The system offers a comprehensive and versatile portfolio for variable-angle plating across various patient populations. It is designed for internal fixation and stabilization of bone fractures, osteotomies, and arthrodesis in normal and osteopenic bone.
Key features of the Pangea Plating System include:
- Evidence-based design for improved implant fit
- Enhanced plate fit and screw placement
- Anatomically contoured implants for diverse fracture patterns
- 20 anatomic plates and 13 utility plates in one platform
- Intuitive and streamlined instrumentation
Stryker will host 'The Pangea Experience' event on August 9-10, 2024, in Las Vegas to showcase this new technology and offer insights to customers.
Stryker (NYSE: SYK) ha lanciato il suo Pangea Plating System, che ha ottenuto l'approvazione della FDA a fine 2023. Il sistema offre un portafoglio completo e versatile per placche a angolo variabile in diverse popolazioni di pazienti. È progettato per il fissaggio interno e la stabilizzazione delle fratture ossee, osteotomie e articolazioni in ossa normali e osteopeniche.
Le principali caratteristiche del Pangea Plating System includono:
- Design basato su evidenze per un miglior adattamento dell'impianto
- Adattamento migliorato della placca e posizionamento delle viti
- Impianti anatomici contornati per diversi schemi di frattura
- 20 placche anatomiche e 13 placche utility su una sola piattaforma
- Strumentazione intuitiva e semplificata
Stryker ospiterà l'evento 'The Pangea Experience' nei giorni 9-10 agosto 2024 a Las Vegas per presentare questa nuova tecnologia e offrire approfondimenti ai clienti.
Stryker (NYSE: SYK) ha lanzado su Sistema de Placas Pangea, que recibió la aprobación de la FDA a finales de 2023. El sistema ofrece un portafolio completo y versátil para placas de ángulo variable en diversas poblaciones de pacientes. Está diseñado para la fijación interna y estabilización de fracturas óseas, osteotomías y artrodesis en huesos normales y osteopénicos.
Las características clave del Sistema de Placas Pangea incluyen:
- Diseño basado en evidencia para un mejor ajuste del implante
- Ajuste mejorado de la placa y colocación de tornillos
- Implantes anatómicamente contorneados para diversos patrones de fractura
- 20 placas anatómicas y 13 placas utilitarias en una sola plataforma
- Instrumentación intuitiva y simplificada
Stryker organizará el evento 'La Experiencia Pangea' los días 9 y 10 de agosto de 2024 en Las Vegas para presentar esta nueva tecnología y ofrecer información a los clientes.
Stryker (NYSE: SYK)는 2023년 말 FDA 승인을 받은 판게아 플레이트 시스템을 출시했습니다. 이 시스템은 다양한 환자 집단에 걸쳐 가변 각도 플레이트를 위한 종합적이고 다재다능한 포트폴리오를 제공합니다. 이는 정상 및 골다공증 뼈의 뼈 골절, 골절 절제술 및 관절 고정의 내부 고정 및 안정화를 위해 설계되었습니다.
판게아 플레이트 시스템의 주요 특징은 다음과 같습니다:
- 임플란트 맞춤 개선을 위한 증거 기반 설계
- 플레이트 적합성 및 나사 배치 향상
- 다양한 골절 패턴을 위한 해부학적으로 윤곽진 임플란트
- 하나의 플랫폼에 20개의 해부학적 플레이트와 13개의 유틸리티 플레이트
- 직관적이고 간소화된 기구
Stryker는 2024년 8월 9-10일 라스베가스에서 이 새로운 기술을 선보이고 고객에게 통찰력을 제공하는 '판게아 경험' 이벤트를 개최할 예정입니다.
Stryker (NYSE: SYK) a lancé son Système de Plaques Pangea, qui a obtenu l'approbation de la FDA fin 2023. Le système propose un portefeuille complet et polyvalent pour des plaques à angle variable pour différentes populations de patients. Il est conçu pour la fixation interne et la stabilisation des fractures osseuses, des ostéotomies et des arthrodèses dans des os normaux et ostéopéniques.
Les caractéristiques clés du Système de Plaques Pangea comprennent :
- Conception basée sur des preuves pour un meilleur ajustement de l'implant
- Amélioration de l'ajustement de la plaque et du placement des vis
- Implants anatomiquement contournés pour divers types de fractures
- 20 plaques anatomiques et 13 plaques utilitaires sur une seule plateforme
- Instrumentation intuitive et optimisée
Stryker organisera l'événement 'The Pangea Experience' les 9 et 10 août 2024 à Las Vegas pour présenter cette nouvelle technologie et offrir des informations aux clients.
Stryker (NYSE: SYK) hat sein Pangea Plattensystem vorgestellt, das Ende 2023 die FDA-Zulassung erhalten hat. Das System bietet ein umfassendes und vielseitiges Portfolio für Platten mit variablen Winkeln für verschiedene Patientengruppen. Es wurde für die interne Fixierung und Stabilisierung von Knochenbrüchen, Osteotomien und Arthrodesen in normalem und osteopenischem Knochen entwickelt.
Die wichtigsten Merkmale des Pangea Plattensystems sind:
- Evidenzbasiertes Design für eine verbesserte Implantatanpassung
- Verbesserte Plattenanpassung und Schraubenplatzierung
- Anatomisch konturierte Implantate für verschiedene Frakturarten
- 20 anatomische Platten und 13 Utility-Platten auf einer Plattform
- Intuitive und vereinfachte Instrumentierung
Stryker wird vom 9. bis 10. August 2024 in Las Vegas die Veranstaltung 'The Pangea Experience' ausrichten, um diese neue Technologie vorzustellen und den Kunden Einblicke zu gewähren.
- FDA clearance received for Pangea Plating System in late 2023
- Comprehensive and versatile portfolio for variable-angle plating
- Designed to enhance plate fit and screw placement
- Offers 20 anatomic plates and 13 utility plates in one platform
- Positive feedback from early adopting surgeons
- None.
Insights
Stryker's launch of the Pangea Plating System represents a significant advancement in trauma care technology. The system's variable-angle plating and comprehensive portfolio address a wide range of patient needs, potentially expanding Stryker's market share in the trauma segment. The
The system's versatility and user-friendly design could lead to increased adoption rates among surgeons, potentially driving sales growth in Stryker's trauma business. However, the competitive landscape in this sector is intense, with players like DePuy Synthes and Zimmer Biomet also offering advanced plating systems. Stryker's success will depend on effectively differentiating Pangea and leveraging its comprehensive trauma portfolio to gain a competitive edge.
The Pangea Plating System's anatomically contoured implants and evidence-based design for implant fit are significant advancements. These features can potentially improve surgical outcomes and reduce operative time, which is important in trauma cases. The system's versatility, with 20 anatomic plates and 13 utility plates in one platform, allows for a wide range of applications, from simple fractures to complex reconstructions.
The variable-angle plating technology is particularly noteworthy, as it provides surgeons with greater flexibility in screw placement, which can be critical in challenging fracture patterns or in patients with poor bone quality. However, the learning curve associated with new systems should be considered, as it may initially impact adoption rates among surgeons comfortable with existing solutions.
New system enhances trauma care for surgeons with reliable and user-friendly solutions
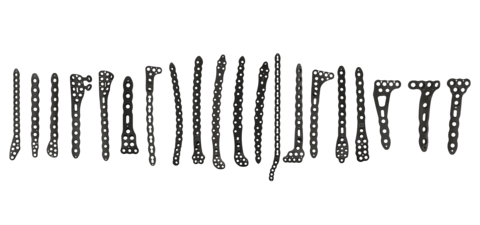
Stryker’s Pangea Plating System is indicated for the internal fixation and stabilization of bone fractures, osteotomies and arthrodesis in normal and osteopenic bone. (Photo: Business Wire)
“The launch of the Pangea Plating System marks a significant milestone for Stryker, our customers and the patients they serve,” said Eric Tamweber, vice president and general manager, Stryker’s Trauma business. “With the addition of Pangea, Stryker is the go-to partner for everything trauma – nails, plates, external fixation devices, and more, backed by our dedicated team and exceptional service.”
Among the 26 pioneering surgeons leading the way with Pangea are:
-
Dr. David A. Forsh, orthopaedic surgeon in
New York, N.Y.
“I am honored to be part of the early adoption of Stryker’s Pangea Plating System. In my recent cases, Pangea demonstrated exceptional performance and versatility, reaffirming my confidence in its potential to elevate trauma care.” -
Dr. Sanjit Konda, chairman of orthopaedic surgery and director of orthopaedic trauma in
Queens, N.Y.
“I am thrilled to have had the opportunity to utilize the Pangea Plating System in my practice. Its intuitive design and reliability make it a valuable addition to our armamentarium for treating traumatic injuries.” -
Dr. Joseph Hsu, board-certified orthopaedic surgeon in
Charlotte, N.C.
“Utilizing Stryker's Pangea Plating System has been a game-changer in my practice. Its innovative design and versatility have allowed me to confidently approach trauma cases, knowing that I have the best tools to provide optimal care for my patients.”
The Pangea Plating System is indicated for the internal fixation and stabilization of bone fractures, osteotomies and arthrodesis in normal and osteopenic bone. Offering an evidence-based design for implant fit, the system was designed to enhance plate fit and screw placement while elevating the plating market through anatomically contoured implants in patient populations with a wide variety of fracture patterns. The intuitive and streamlined instrumentation and implant trays include 20 anatomic plates and 13 utility plates all accessible in one platform.
Stryker will host The Pangea Experience for its customers on Aug. 9 and 10, 2024, at the MGM Grand in
About Stryker
Stryker is a global leader in medical technologies and, together with its customers, is driven to make healthcare better. The company offers innovative products and services in MedSurg, Neurotechnology, Orthopaedics and Spine that help improve patient and healthcare outcomes. Alongside its customers around the world, Stryker impacts more than 150 million patients annually. More information is available at www.stryker.com.
This document is intended solely for healthcare professionals. A surgeon must always rely on his or her own professional clinical judgment when deciding whether to use a particular product when treating a particular patient. Stryker does not dispense medical advice and recommends that surgeons be trained in the use of any particular product before using it in surgery.
The information presented is intended to demonstrate the breadth of Stryker’s product offerings. A surgeon must always refer to the package insert, product label and/or instructions for use before using any of Stryker’s products. Products may not be available in all markets because product availability is subject to the regulatory and/or medical practices in individual markets. Please contact your sales representative if you have questions about the availability of products in your area.
Stryker Corporation or its affiliates own, use, or have applied for the following trademarks or service marks: Stryker. All other trademarks are trademarks of their respective owners or holders.
Content ID: PGA-AR-2_35828
View source version on businesswire.com: https://www.businesswire.com/news/home/20240806005748/en/
Andrea Sampson
President/CEO, Sampson Public Relations Group
asampson@sampsonprgroup.com
Source: Stryker
FAQ
What is Stryker's Pangea Plating System and when was it FDA cleared?
What are the main indications for Stryker's Pangea Plating System (SYK)?
How many plate types does Stryker's Pangea Plating System offer?






