Sorrento Announces License From Columbia University For Rapid On-Site Detection Test For SARS-CoV-2 Virus In Saliva
Sorrento Therapeutics (Nasdaq: SRNE) has licensed a rapid diagnostic test from Columbia University for SARS-CoV-2 detection in saliva, marketed as COVI-TRACE. This test provides results in 30 minutes without the need for specialized laboratory equipment, addressing the current backlog in testing that delays results for days. The test demonstrates a sensitivity of 97% and 100% specificity in initial studies. Sorrento aims to expedite Emergency Use Authorization (EUA) submission to the FDA, positioning COVI-TRACE as a vital tool in combating COVID-19.
- Licensing of COVI-TRACE test for rapid SARS-CoV-2 detection from Columbia University.
- Test results in 30 minutes with 97% sensitivity and 100% specificity.
- No specialized laboratory equipment required, enhancing deployability for on-site testing.
- Sorrento plans to submit EUA to the FDA quickly for COVI-TRACE.
- None.
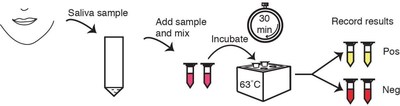
SAN DIEGO, July 29, 2020 /PRNewswire/ -- Sorrento Therapeutics, Inc. (Nasdaq: SRNE) ("Sorrento") today announced it has entered into a licensing agreement with Columbia University ("Columbia") for the rights to a rapid, one-step diagnostic test that detects SARS-CoV-2 virus in as little as 30 minutes from a sample of saliva. Unlike other commercially available diagnostic products, the test developed by Columbia's team, to be marketed by Sorrento under the COVI-TRACE™ name, holds all of the testing materials in a single tube and requires no specialized laboratory equipment, making it easily deployable for point of care, on-site or potentially at-home testing.
Current diagnostic tests for SARS-CoV-2 detect viral ribonucleic acid (RNA) but must be shipped to a reference laboratory unless the facility collecting the samples has purchased costly instrumentation, cartridges and consumables to extract viral RNA from the fluid in which the sample — either a nasopharyngeal swab or saliva — is placed. The current backlog in SARS-CoV-2 testing has resulted in average turnaround times of between several days to over a week, and laboratories across the country are reportedly struggling to keep up with increased testing demand.
Developed by Zev Williams, MD, PhD, and his team at the Columbia University Fertility Center, the COVI-TRACE approach eliminates the extraction step and simplifies overall sample processing. A small sample of saliva is collected in a cup and then placed into a tube containing enzymes and reagents that can detect the SARS-CoV-2 virus's RNA. The tube is then placed into a simple heat block or water bath to keep the sample warm throughout the chemical reaction, which takes 30 minutes or less to provide a colorimetric reading based on detection of the presence of the virus.
Preliminary study results were published in a paper by Williams and colleagues at Columbia University Irving Medical Center titled, "Field-deployable, rapid and direct diagnostic testing of saliva samples for SARS-CoV-2," in MedRxiv on June 16, 2020. The study evaluated the new test in 60 samples, including 30 samples with virus and 30 without. The study found sensitivity and specificity of
Dr. Zev Williams, Director of the Columbia University Fertility Center, stated, "Testing for SARS-CoV-2 needs to be fast, frequent, and far-reaching. We are delighted to work with Sorrento Therapeutics in the hope that COVI-TRACE may be scaled and deployed in the U.S. and around the world to combat the spread of COVID-19."
Dr. Henry Ji, Chairman and CEO of Sorrento Therapeutics, Inc., stated, "We are building a portfolio of highly relevant COVID-19 solutions that spans diagnostics, prevention, early intervention and rescue therapies. COVI-TRACE will be a key asset in our diagnostic solutions, and we intend to move rapidly to submit an emergency use authorization request to the FDA and prepare for full-scale production. Such a simple, deployable and cost-effective solution, in synergy with our potentially neutralizing antibodies, could become the 'economy opener' our country has been waiting for."
Reading test results is designed to be simple (Figure 1): If the fluid in the tube turns yellow, the test is considered positive (virus RNA present), and if the fluid turns red, the test is considered negative (no virus RNA present).
About Sorrento Therapeutics, Inc.
Sorrento is a clinical stage, antibody-centric, biopharmaceutical company developing new therapies to treat cancers. Sorrento's multimodal, multipronged approach to fighting cancer is made possible by its extensive immuno-oncology platforms, including key assets such as fully human antibodies ("G-MAB™ library"), clinical stage immuno-cellular therapies ("CAR-T", "DAR-T™"), antibody-drug conjugates ("ADCs"), and clinical stage oncolytic virus ("Seprehvir™", "Seprehvec™"). Sorrento is also developing potential antiviral therapies and vaccines against coronaviruses, including COVIDTRAP™, ACE-MAB™, COVI-MAB™, COVI-GUARD™, COVI-SHIELD™ and T-VIVA-19™; and diagnostic test solutions, including COVI-TRACK™ and COVI-TRACE™.
Sorrento's commitment to life-enhancing therapies for patients is also demonstrated by our effort to advance a first-in-class (TRPV1 agonist) non-opioid pain management small molecule, resiniferatoxin ("RTX"), and ZTlido® (lidocaine topical system)
For more information visit www.sorrentotherapeutics.com
Forward-Looking Statements
This press release and any statements made for and during any presentation or meeting contain forward-looking statements related to Sorrento Therapeutics, Inc., under the safe harbor provisions of Section 21E of the Private Securities Litigation Reform Act of 1995 and subject to risks and uncertainties that could cause actual results to differ materially from those projected. Forward-looking statements include statements regarding potential deployment points for COVI-TRACE; COVI-TRACE's ability to detect the SARS-CoV-2 virus; the receipt of emergency use authorization (EUA) approval for COVI-TRACE; the expected regulatory, development and commercialization path for COVI-TRACE; the readiness of Sorrento for full-scale production of COVI-TRACE; the cost-effectiveness of COVI-TRACE; the neutralizing effects of Sorrento's antibodies; the impact that Sorrento's products and product candidates may have on the U.S. economy; Sorrento's pipeline of product candidates for the potential detection, vaccination and treatment of COVID-19; and Sorrento's potential position in the anti-viral immunity industry. Risks and uncertainties that could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements, include, but are not limited to: risks related to Sorrento's and its subsidiaries', affiliates' and partners' technologies and prospects and collaborations with partners, including, but not limited to risks related to seeking EUA regulatory approval for COVI-TRACE; the clinical and commercial success of the detection of the SARS-CoV-2 virus infections using COVI-TRACE; the viability and success of using COVI-TRACE for detection of the SARS-CoV-2 virus; clinical development risks, including risks in the progress, timing, cost, and results of clinical trials and product development programs; risk of difficulties or delays in obtaining regulatory approvals; risks that clinical study results may not meet any or all endpoints of a clinical study and that any data generated from such studies may not support a regulatory submission or approval; risks of manufacturing and supplying drug product; risks related to leveraging the expertise of its employees, subsidiaries, affiliates and partners to assist the company in the execution of its COVI-TRACE strategies; risks related to the global impact of COVID-19; and other risks that are described in Sorrento's most recent periodic reports filed with the Securities and Exchange Commission, including Sorrento's Annual Report on Form 10-K for the year ended December 31, 2019, and subsequent Quarterly Reports on Form 10-Q filed with the Securities and Exchange Commission, including the risk factors set forth in those filings. Investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this release and we undertake no obligation to update any forward-looking statement in this press release except as required by law.
Media and Investor Relations
Contact: Dani Frank
Email: sorrentotherapeutics@factorypr.com
Sorrento® and the Sorrento logo are registered trademarks of Sorrento Therapeutics, Inc.
G-MAB™, COVI-GUARD™, COVI-SHIELD™, COVIDTRAP™, T-VIVA-19™, COVI-MAB™, ACE-MAB™, COVI-TRACK™, COVI-TRACE™, Saving-Life™ and Improving-Life™ are trademarks of Sorrento Therapeutics, Inc.
ZTlido® is a trademark owned by Scilex Pharmaceuticals Inc.
All other trademarks are the property of their respective owners.
© 2020 Sorrento Therapeutics, Inc. All Rights Reserved.
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/sorrento-announces-license-from-columbia-university-for-rapid-on-site-detection-test-for-sars-cov-2-virus-in-saliva-301102044.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/sorrento-announces-license-from-columbia-university-for-rapid-on-site-detection-test-for-sars-cov-2-virus-in-saliva-301102044.html
SOURCE Sorrento Therapeutics, Inc.
FAQ
What is the COVI-TRACE test announced by Sorrento Therapeutics?
What are the sensitivity and specificity rates of the COVI-TRACE test?
When did Sorrento Therapeutics announce the licensing of the COVI-TRACE test?
How does the COVI-TRACE test differ from current SARS-CoV-2 tests?