ARS Pharmaceuticals Announces U.S. Availability of neffy® (epinephrine nasal spray), the First and Only Needle-Free Treatment for Type I Allergic Reactions, Including Anaphylaxis
Rhea-AI Summary
ARS Pharmaceuticals (Nasdaq: SPRY) announced that neffy® (epinephrine nasal spray) is now available by prescription across the U.S. for treating Type I Allergic Reactions, including anaphylaxis, in adults and children weighing ≥30 kg. The FDA approved neffy 2 mg last month, marking it as the first and only needle-free treatment for severe allergic reactions.
Through neffyConnect and BlinkRx, eligible commercially insured patients can obtain two single-use neffy devices for a $25 co-pay. Uninsured patients or those without coverage can access neffy for $199 for two devices. The company is also offering free carrying cases and has implemented various patient assistance programs to ensure accessibility.
ARS Pharmaceuticals plans to expand access to EURneffy® in Europe and has submitted an sNDA for neffy 1 mg use in pediatric patients weighing 15 to 30 kg.
Positive
- First and only FDA-approved needle-free epinephrine treatment for severe allergic reactions
- Now available by prescription across the U.S.
- Affordable pricing options: $25 co-pay for eligible insured patients, $199 for uninsured patients
- European Commission approval for EURneffy obtained
- Potential market expansion with sNDA submission for pediatric use (15-30 kg)
Negative
- None.
News Market Reaction 1 Alert
On the day this news was published, SPRY declined 4.60%, reflecting a moderate negative market reaction.
Data tracked by StockTitan Argus on the day of publication.
Visit neffy.com to “Get neffy Now” and access the comprehensive patient assistance programs available through neffyConnect
SAN DIEGO, Sept. 23, 2024 (GLOBE NEWSWIRE) -- ARS Pharmaceuticals, Inc. (Nasdaq: SPRY), a biopharmaceutical company dedicated to empowering at-risk patients and their caregivers to better protect patients from severe allergic reactions that could lead to anaphylaxis, announced today that neffy® (epinephrine nasal spray) is now available to patients and caregivers by prescription across the U.S. The U.S. Food and Drug Administration (FDA) approved neffy 2 mg last month for the treatment of Type I Allergic Reactions, including anaphylaxis, in adults and children who weigh ≥30 kg (66 lbs.).

Through neffyConnect, and in partnership with online pharmacy BlinkRx, eligible commercially insured patients will pay
“The introduction of neffy as the first and only needle-free treatment option for patients and caregivers living with severe allergic reactions marks a turning point for ARS Pharmaceuticals and the severe allergy community. Since approval, we have been partnering with healthcare providers, payers, and patient advocates to ensure access for patients,” said Eric Karas, chief commercial officer of ARS Pharmaceuticals. “Pharmacies are processing prescriptions, our supply chain is fully operational, and most importantly, the first patients are now receiving neffy.”

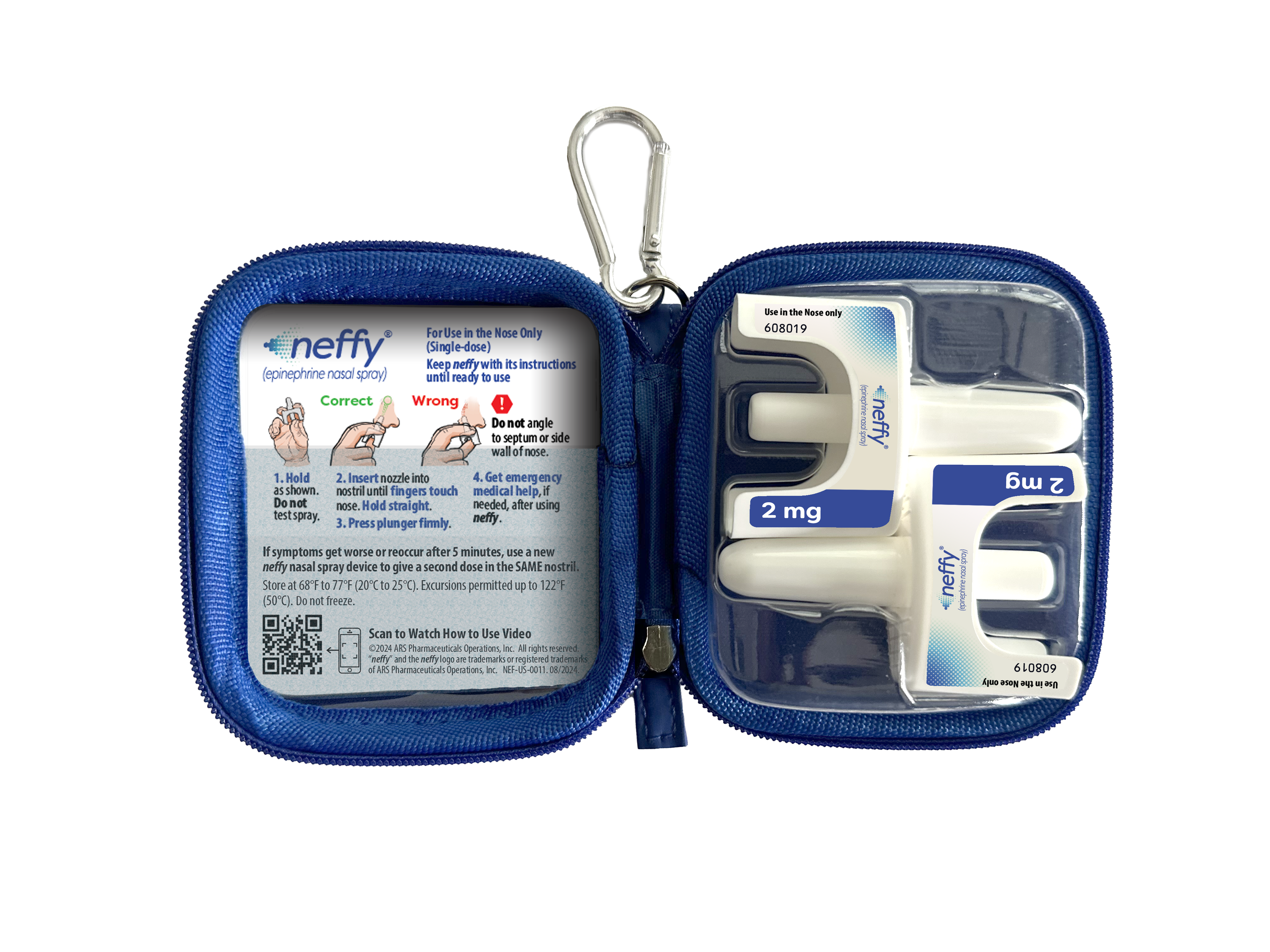
In addition to scheduling an appointment with healthcare providers to prescribe neffy, other options for patients who need to obtain a prescription include visiting neffy.com and selecting the “Get neffy Now” button. They can request neffy either through their existing healthcare provider or via a virtual consultation with a licensed physician. Both options are supported by neffyConnect, a service designed to help patients with access and financial assistance. ARS Pharmaceuticals is also offering free carrying cases that hold two single-use neffy devices with instructions for use, encouraging having neffy conveniently available when needed.
“The introduction of neffy is welcomed by the healthcare community,” said Dr. Carlos Camargo, MD DrPH, Professor of Emergency Medicine at Massachusetts General Hospital and Harvard Medical School. “neffy changes the paradigm when it comes to treatment options for patients and families living with severe allergic reactions — and the way in which healthcare providers practice. Epinephrine auto-injectors have needles and this can be intimidating for patients. This can lead to hesitancy in carrying and in using the life-saving devices, and often results in patients failing to treat before seeking emergency medical assistance. neffy provides a fast, easy-to-carry, easy-to-administer alternative that we anticipate will result in more people carrying their epinephrine device and treating their allergic event earlier. These changes are likely to lead to better clinical outcomes and less need for emergency room visits.”
“Years of dedication from the entire allergy community and healthcare providers have paved the way for neffy as the first needle-free epinephrine treatment for severe allergies,” said Richard Lowenthal, Co-Founder, President, and CEO of ARS Pharmaceuticals. “We are so proud of our team members who have worked diligently from conception through FDA approval to make neffy available. Now that people can begin using neffy, we are looking forward to seeing how it positively impacts up to 40 million people in the U.S. who experience severe allergic reactions.”
Expanding Access in Europe, Pediatric Indications
In addition to its U.S. launch, ARS Pharmaceuticals plans to expand access to EURneffy® (trade name for neffy in the EU) internationally in partnership with an EU-based pharmaceutical partner. On August 22, 2024, the European Commission approved EURneffy (adrenaline nasal spray) for the emergency treatment of allergic reactions (anaphylaxis) in the EU. The company expects EURneffy to be available in certain EU Member States by Q4 2024.
ARS Pharmaceuticals has also submitted a supplemental New Drug Application (sNDA) to the FDA for the use of neffy 1 mg in pediatric patients weighing 15 to 30 kg (33 to 66 lbs.), further broadening its potential to protect vulnerable populations from severe allergic reactions.
About Type I Allergic Reactions including Anaphylaxis
Type I severe allergic reactions are serious and potentially life-threatening events that can occur within minutes of exposure to an allergen and require immediate treatment with epinephrine, the only FDA-approved medication for these reactions. While epinephrine auto-injectors have been shown to be highly effective, there are well published limitations that result in many patients and caregivers delaying or not administering treatment in an emergency situation. These limitations include fear of the needle, lack of portability, needle-related safety concerns, lack of reliability, and complexity of the devices. There are approximately 40 million people in the United States who experience Type I severe allergic reactions due to food, venom or insect stings. Of those, only 3.3 million currently have an active epinephrine auto-injector prescription, and of those, only half consistently carry their prescribed auto-injector. Even if patients or caregivers carry an auto-injector, more than half either delay or do not administer the device when needed in an emergency.
About neffy®
neffy® is an intranasal epinephrine product for patients with Type I allergic reactions including food, medications, and insect bites that could lead to life-threatening anaphylaxis.
INDICATION AND IMPORTANT SAFETY INFORMATION FOR neffy (epinephrine nasal spray)
INDICATION
neffy 2 mg is indicated for emergency treatment of Type I allergic reactions, including anaphylaxis, in adult and pediatric patients who weigh 30 kg or greater.
IMPORTANT SAFETY INFORMATION
It is recommended that patients are prescribed and have immediate access to two neffy nasal sprays at all times. In the absence of clinical improvement or if symptoms worsen after initial treatment, administer a second dose of neffy in the same nostril with a new nasal spray starting 5 minutes after the first dose.
neffy is for use in the nose only.
Advise patients when to seek emergency medical assistance for close monitoring of the anaphylactic episode and in the event further treatment is required.
Absorption of neffy may be affected by underlying structural or anatomical nasal conditions.
Administer with caution to patients who have heart disease; epinephrine may aggravate angina pectoris or produce ventricular arrhythmias. Arrhythmias, including fatal ventricular fibrillation, have been reported, particularly in patients with underlying cardiac disease or taking cardiac glycosides, diuretics, or anti-arrhythmics.
The presence of a sulfite in neffy should not deter use.
neffy may alter nasal mucosa for up to 2 weeks after administration and increase systemic absorption of nasal products, including neffy.
Patients with certain medical conditions or who take certain medications for allergies, depression, thyroid disorders, diabetes, and hypertension, may be at greater risk for adverse reactions.
Epinephrine can temporarily exacerbate the underlying condition or increase symptoms in patients with the following: hyperthyroidism, Parkinson’s disease, diabetes, renal impairment. Epinephrine should be administered with caution in patients with these conditions, including elderly patients and pregnant women.
Adverse reactions to neffy may include throat irritation, intranasal paresthesia, headache, nasal discomfort, feeling jittery, paresthesia, fatigue, tremor, rhinorrhea, nasal pruritus, sneezing, abdominal pain, gingival pain, hypoesthesia oral, nasal congestion, dizziness, nausea, and vomiting.
These are not all of the possible side effects of neffy. To report suspected adverse reactions, contact ARS Pharmaceuticals Operations, Inc. at 1-877-MY-NEFFY (877-696-3339) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
For additional information on neffy, please see Full Prescribing Information at www.neffy.com.
About ARS Pharmaceuticals, Inc. ARS Pharmaceuticals is a biopharmaceutical company dedicated to empowering at-risk patients and their caregivers to better protect patients from severe allergic reactions that could lead to anaphylaxis. The Company is commercializing neffy® 2 mg (trade name EURneffy® in the EU) (previously referred to as ARS-1), an epinephrine nasal spray indicated in the US for emergency treatment of Type I allergic reactions, including anaphylaxis, in adult and pediatric patients who weigh 30 kg or greater, and in the EU for emergency treatment of allergic reactions (anaphylaxis) due to insect stings or bites, foods, medicinal products and other allergens as well as idiopathic or exercise induced anaphylaxis in adults and children who weigh 30 kg or greater. For more information, visit www.ars-pharma.com.
Forward-Looking Statements
Statements in this press release that are not purely historical in nature are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. These statements include but are not limited to: the platforms through which neffy will be accessible, including BlinkRx; neffy, neffy.com, and neffyConnect’s potential benefits to patients and caregivers; the needle-free profile of neffy potentially increasing the likelihood that patients may both carry and administer epinephrine; the potential market and demand for neffy; the timelines for commercialization of EURneffy in the European Union; ARS Pharmaceuticals’s marketing and commercialization strategies, including potential partnerships in foreign jurisdictions; and other statements that are not historical fact. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Words such as “can,” “could,” “expects,” “likely,” “may,” “plans,” “potential,” “will,” and similar expressions are intended to identify forward-looking statements. These forward-looking statements are based upon ARS Pharmaceuticals’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation: the ability to obtain and maintain regulatory approval for neffy; potential safety and other complications from neffy; the labelling for neffy in any future indication or patient population, if approved; the scope, progress and expansion of developing and commercializing neffy; the potential for payors to delay, limit or deny coverage for neffy; the size and growth of the market therefor and the rate and degree of market acceptance thereof vis-à-vis intramuscular injectable products; ARS Pharmaceuticals’s ability to protect its intellectual property position; and the impact of government laws and regulations. Additional risks and uncertainties that could cause actual outcomes and results to differ materially from those contemplated by the forward-looking statements are included under the caption “Risk Factors” in ARS Pharmaceuticals’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2024, filed with the Securities and Exchange Commission (SEC) on August 6, 2024. This and other documents ARS Pharmaceuticals files with the SEC can also be accessed on ARS Pharmaceuticals’s website at ir.ars-pharma.com by clicking on the link “Financials & Filings” under the “Investors & Media” tab.
ARS Investor Contact:
Justin Chakma
ARS Pharmaceuticals
justinc@ars-pharma.com
ARS Media Contact:
Christy Curran
Sam Brown Inc.
615.414.8668
christycurran@sambrown.com
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/569986d5-31e4-43ca-81db-f86a140ea9c7
https://www.globenewswire.com/NewsRoom/AttachmentNg/6d8266f8-1a81-4eed-a8e1-61cbf375afb1
https://www.globenewswire.com/NewsRoom/AttachmentNg/7a549d27-68ee-4345-91de-b97663ea1d75









