ARS Pharmaceuticals announces neffy® meets primary endpoints and shows rapid symptom control in Phase 2 urticaria clinical study
- Positive efficacy results in phase 2 study for neffy in treating chronic spontaneous urticaria.
- Statistically significant improvements in itch, hives, and erythema scores.
- Rapid onset of action observed as early as 5 minutes after dosing.
- Plans for outpatient study in acute flares in 2024 and potential pivotal study in 2025.
- Impressive results in refractory patient population with chronic urticaria.
- Neffy offers a potential novel treatment option for non-responsive patients.
- No serious adverse events reported, with mild to moderate adverse events.
- Neffy could address existing gaps in efficacy of currently available treatments.
- Urticaria affects approximately 5 million cases in the US annually.
- Less than 40% of cases become chronic urticaria, with many non-responsive to antihistamines.
- None.
Insights
Chronic spontaneous urticaria (CSU) represents a challenging condition for both patients and the healthcare system, often requiring long-term management and treatment strategies. The recent findings from ARS Pharmaceuticals' phase 2 study of neffy, an epinephrine nasal spray, are of significant interest due to the potential impact on treatment paradigms for CSU. The study's positive results, showing efficacy in reducing pruritus, hives, body surface area and erythema, suggest that neffy may address the unmet needs of patients who are resistant to first-line antihistamines and biologics.
The implications for stakeholders are multifaceted. For patients, the availability of a rapid-onset, non-invasive treatment could greatly improve quality of life and reduce the burden of disease. For healthcare providers, neffy could offer a novel intervention that may reduce the frequency of acute flare management, including emergency room visits. For ARS Pharmaceuticals, the successful development of neffy may open up a new market segment within the allergy therapeutics space, potentially leading to increased revenue streams and market share. However, it is important to consider the long-term safety profile of neffy, as well as its cost-effectiveness compared to existing treatments.
The clinical significance of these findings is underscored by the fact that nearly half of CSU cases do not respond to first-line therapies, highlighting the importance of developing alternative treatments. The absence of serious adverse events in the study is promising, although the long-term safety and tolerability of neffy remain to be established in larger patient populations and over extended periods of use.
From an economic perspective, the development of neffy could have considerable implications for the healthcare system. CSU is associated with a substantial economic burden, including direct medical costs and indirect costs related to loss of productivity. Innovative treatments like neffy that offer rapid relief could potentially reduce the direct costs associated with managing acute flares, such as hospital admissions and outpatient visits.
Furthermore, the potential for neffy to be used as an outpatient treatment aligns with the broader trend in healthcare towards cost containment and the shift from inpatient to outpatient care settings. By potentially reducing the need for emergency room visits and hospitalizations, neffy could contribute to lowering healthcare expenditures. However, the pricing strategy for neffy will be a critical factor in its economic impact. If priced competitively with existing therapies, neffy could be widely adopted, whereas a high price point might limit its use to only the most severe cases or lead to payer restrictions.
Investors and market analysts will monitor the progress of neffy closely, as its market penetration will depend not only on its clinical efficacy and safety but also on reimbursement policies and the competitive landscape. The planned pivotal study in 2025 will be a key milestone to watch, as it will provide more robust data that can influence payer decisions and market uptake.
The announcement of neffy's phase 2 trial results is likely to have a positive impact on ARS Pharmaceuticals' stock performance in the short term, as investors often react favorably to positive clinical trial outcomes. The potential initiation of a pivotal study in 2025 may further bolster investor confidence if the development timeline is adhered to and no significant hurdles emerge.
It's important to note that the CSU treatment market is characterized by a high demand for new therapies due to the limited efficacy of current options for a subset of patients. As a result, neffy's market entry could be met with significant interest from both prescribers and patients. The differentiation of neffy as a non-invasive, rapid-onset treatment is likely to be a key selling point. However, the competitive dynamics of the market, including the presence of biologics and other novel therapies, will influence neffy's market positioning and pricing strategy.
Analyzing the company's capacity to meet potential demand, manufacturing scalability and distribution channels will be important in assessing the long-term business implications. Additionally, intellectual property considerations, such as patent protection and potential for market exclusivity, will play a role in the financial outlook for neffy and ARS Pharmaceuticals.
- neffy demonstrated statistically significant and clinically meaningful improvement in pruritus, hives, body surface area and erythema in treatment-resistant chronic spontaneous urticaria patients, a skin disorder that causes itchy hives and/or angioedema
- Data supports continued development of neffy in urticaria with planned outpatient study in acute flares to initiate during 2024, with pivotal study to potentially initiate in 2025
SAN DIEGO, Feb. 26, 2024 (GLOBE NEWSWIRE) -- ARS Pharmaceuticals, Inc. (NASDAQ: SPRY), a biopharmaceutical company dedicated to empowering at-risk patients and caregivers to better protect patients from severe allergic reactions that could lead to anaphylaxis, today announced positive efficacy results in its phase 2 inpatient chronic spontaneous urticaria study with neffy (epinephrine nasal spray), an investigational new drug. The trial met its primary endpoints with both 1 mg and 2 mg neffy demonstrating statistically significant and clinically meaningful changes from baseline in itch, hives, urticaria and erythema scores as early as 5 minutes after dosing. Urticaria is a skin disorder that causes itchy hives and/or angioedema;
These data for neffy are being presented in an oral presentation today, February 26, at 1:10 pm ET at the 2024 American Academy of Allergy, Asthma and Immunology (AAAAI) in Washington, D.C. in Room 207B (Level 2) of the Convention Center.
“The Phase 2 results in this highly refractory patient population are impressive and encouraging as they indicate neffy could be a game-changing therapeutic advance in the treatment of urticaria,” says David Bernstein, M.D., Emeritus Professor of Pediatrics at the Cincinnati Children’s Hospital Medical Center, a former member of the Joint Task Force on Practice Parameters (urticaria guidelines) and Principal Investigator of the Study, “Urticaria symptoms clear within minutes after dosing, and neffy could offer patients a novel treatment option to address existing gaps in the efficacy of currently available antihistamines or biologics.”
Design of neffy efficacy study in urticaria
The EPI-U01 study was a randomized, placebo-controlled, cross-over study evaluating the safety and efficacy of epinephrine nasal spray in patients with chronic spontaneous urticaria (CSU) treated with chronic medications who were still experiencing flares. This oral presentation includes data analysis from 18 adult patients as of the cut-off date who enrolled in this study and returned to the clinical site while experiencing a flare, where they were randomized to receive either a single treatment of 1 mg or 2 mg neffy or placebo in a crossover design.
All subjects had flares with pruritus and hives scores greater than or equal to 2 on a 3 point severity scale, despite all patients having been treated with antihistamines or Xolair.
Results from neffy efficacy study in urticaria
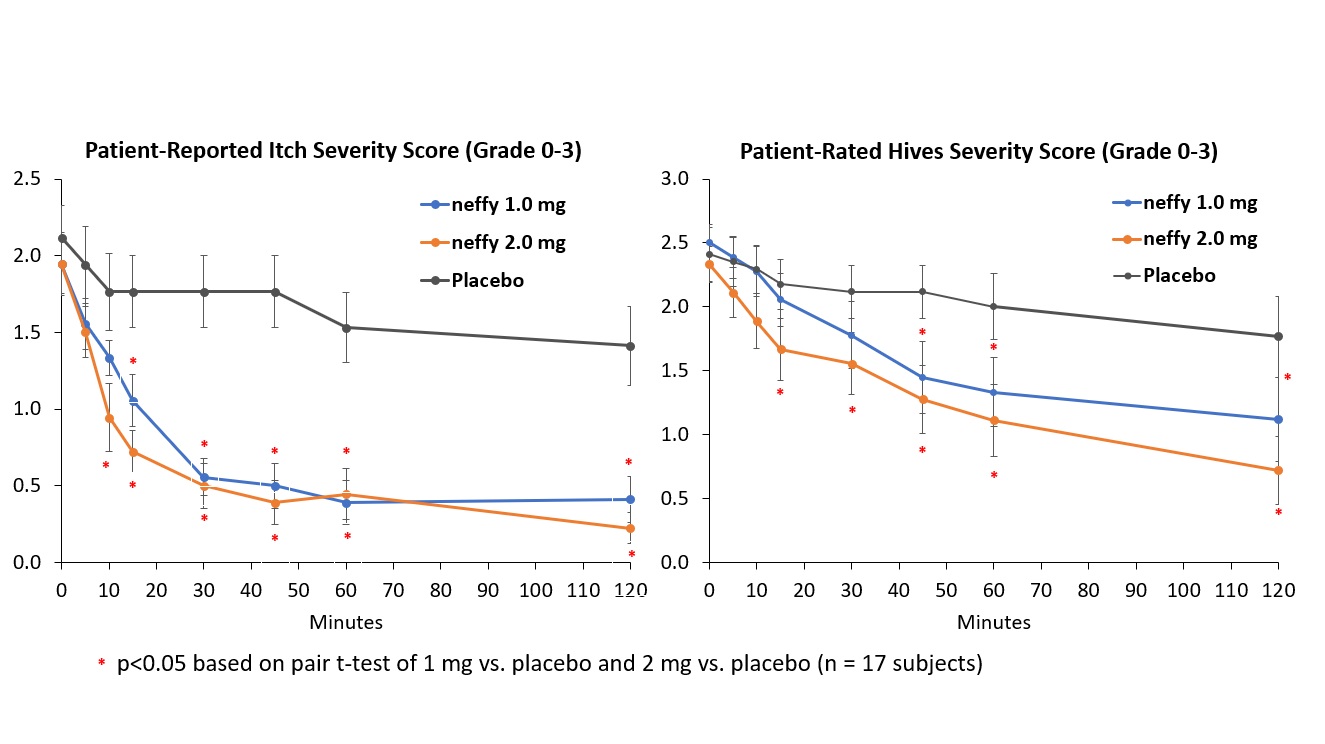
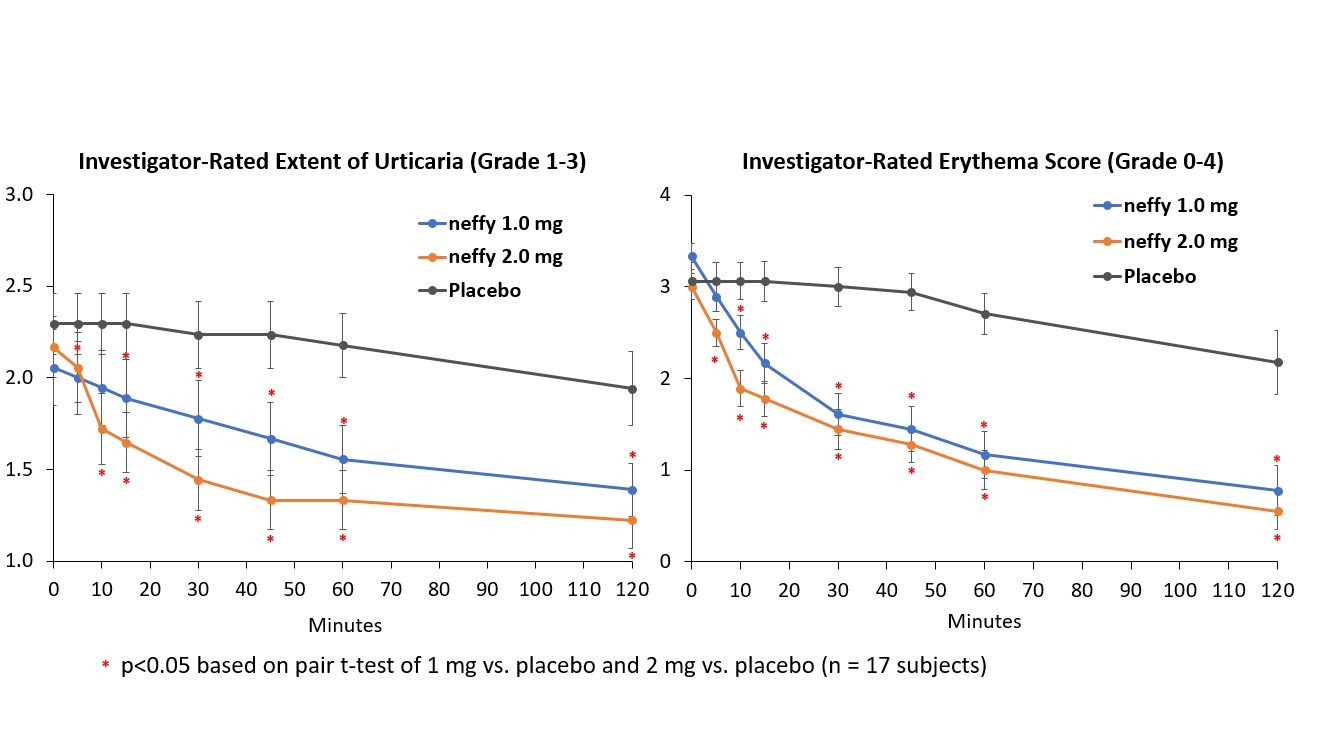
There was no meaningful difference in efficacy on patient-reported itch severity score, patient-reported hives severity score, investigator-related extent of urticaria, or investigator-related erythema score between 1 mg and 2 mg neffy doses, indicating that the 1 mg dose may be sufficient to activate the beta-2 adrenergic receptors responsible for stopping the mast cell degranulation and allergic mediator release that leads to an urticaria flare.
neffy was well-tolerated, with adverse events reported in 8 subjects, which were all mild or moderate in severity. The most common adverse event reported was nasal discomfort in 5 subjects. There were no serious adverse events.
“This study adds to the wealth of data supporting the efficacy and safety of neffy in the treatment of type I allergic reactions,” said Sarina Tanimoto, M.D., Ph.D., Chief Medical Officer and Co-Founder of ARS Pharma. “Urticaria is not only a standalone type I allergy disease, but also represents the most frequent symptom observed during type I allergic reactions including anaphylaxis. The improvement in urticaria symptoms almost immediately after dosing neffy demonstrated its rapid onset of action, and is consistent with the previously reported responses on pharmacodynamic markers of efficacy in as little as one minute after dosing.”
Unmet need in urticaria and role of neffy
Urticaria is a skin disorder driven by mast cell degranulation and histamine release, which causes itchy wheals (hives) and angioedema, or both. In the United States, the annualized incidence is approximately 5 million cases (1.56 per 100,000).2 Less than
Antihistamines are the first-line therapy for treatment of urticaria, but 20 to
Next steps for neffy development in urticaria
ARS Pharma plans to initiate a placebo-controlled outpatient urticaria study in patients treated with antihistamines who experience frequent acute flares later in 2024 followed by the potential initiation of a single pivotal efficacy study in 2025. This would follow the anticipated FDA approval of neffy for allergic reactions (Type I) including anaphylaxis in the second half of 2024.
About Type I Allergic Reactions including Anaphylaxis
Type I severe allergic reactions are serious and potentially life-threatening events that can occur within minutes of exposure to an allergen and require immediate treatment with epinephrine, the only FDA-approved medication for these reactions. While epinephrine autoinjectors have been shown to be highly effective, there are well published limitations that result in many patients and caregivers delaying or not administering treatment in an emergency situation. These limitations include fear of the needle, lack of portability, needle-related safety concerns, lack of reliability, and complexity of the devices. There are approximately 40 million people in the United States who experience Type I severe allergic reactions. Of those, only 3.3 million currently have an active epinephrine autoinjector prescription, and of those, only half consistently carry their prescribed autoinjector. Even if patients or caregivers carry an autoinjector, more than half either delay or do not administer the device when needed in an emergency.
About ARS Pharmaceuticals, Inc.
ARS Pharma is a biopharmaceutical company dedicated to empowering at-risk patients and caregivers to better protect themselves from severe allergic reactions that could lead to anaphylaxis. The Company is developing neffy® (previously referred to as ARS-1), an intranasal epinephrine product in clinical development for patients and their caregivers with Type I allergic reactions including food, medications and insect bites that could lead to life-threatening anaphylaxis. For more information, visit www.ars-pharma.com.
Forward-Looking Statements
Statements in this press release that are not purely historical in nature are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. These statements include, but are not limited to ARS Pharma’s planned urticaria outpatient study and the timing thereof; the potential pivotal study and the timing thereof; neffy potentially being a game-changing therapeutic advance in the treatment of urticaria and offering patients a treatment option to address existing gaps in the efficacy of currently available antihistamines or biologics; 1 mg dose of neffy potentially being sufficient to activate the beta-2 adrenergic receptors; the potential approval of neffy and the timing thereof; and other statements that are not historical fact. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Words such as “anticipate,” “could,” “indicate,” “may,” “plans,” “will,” “potential” and similar expressions are intended to identify forward-looking statements. These forward-looking statements are based upon ARS Pharma’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, the ability to obtain and maintain regulatory approval for neffy; the results of the clinical trials may not support the approval of neffy; results from clinical trials may not be indicative of results that may be observed in the future; potential safety and other complications from neffy; the labelling for neffy, if approved; ARS Pharma’s ability to protect its intellectual property position; and the impact of government laws and regulations. Additional risks and uncertainties that could cause actual outcomes and results to differ materially from those contemplated by the forward-looking statements are included under the caption “Risk Factors” in ARS Pharma’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2023, filed with the Securities and Exchange Commission on November 9, 2023. This document can also be accessed on ARS Pharma’s web page at ir.ars-pharma.com by clicking on the link “Financials & Filings” under the “Investors & Media” tab.
The forward-looking statements included in this press release are made only as of the date hereof. ARS Pharma assumes no obligation and does not intend to update these forward-looking statements, except as required by law.
ARS Media Contacts:
Laura O’Neill
Finn Partners
Laura.oneill@finnpartners.com
ARS Investor Contacts:
Justin Chakma
ARS Pharmaceuticals
justinc@ars-pharma.com
References:
1 Sanchez-Borges M et al. World Allergy Organization Journal 2014; 7, 1-5, Williams, PV et al. Dermatol Ther 2018; 8(1):69-83, Maurer M et al. Clin Exp Allergy 2019; 49(5): 655-662
2 Kolkhir,P. et. al. Urticaria. Nat Rev Dis Primers 8, 61 (2022)
3 Seo JH & Kwon JW. Korean J Intern Med 2019; 34(2):418-425, Sanchez-Borges M et al. Allergologia et Immunopathologia 2015; 43(4): 409-415, Barniol C et al. Annals of Emergency Medicine 2018; 71(1): 125-131
4 Raciborski F, et al. Postepy Dermatol Allerg 2018; 35(1):67-73
5 Barniol C et al. Annals of Emergency Medicine 2018; 71(1): 125-131
6 Sussman G et al. Allergy 2018; 73(8): 1724-1734.
Photos accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/f7f1b49f-1a9d-4ba8-a389-643fc29729be
https://www.globenewswire.com/NewsRoom/AttachmentNg/b3d077c2-eb70-4da8-b291-326bb965da92








