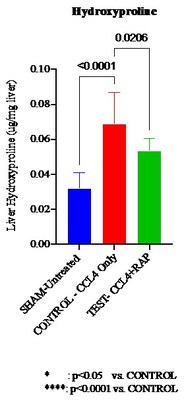
Soliton Announces RAP Treatment Reduces Effects of Liver Fibrosis by 42% in Animal Study
Soliton, Inc. (Nasdaq: SOLY) announced positive pre-clinical results demonstrating that its Rapid Acoustic Pulse (RAP) therapy reduced liver fibrosis by 42% in a mouse model. This study validates the RAP mechanism while exploring treatment for a significant unmet medical need, as current treatments for liver fibrosis are lacking. The global market for liver fibrosis treatment is projected to reach US$ 28.1 billion by 2026. The RAP device is cleared by the FDA for cellulite and tattoo removal, with plans for commercial launch in the first half of 2021.
- RAP therapy demonstrated a 42% reduction in liver fibrosis in a mouse model.
- Positive validation of RAP's mechanism of action for multiple fibrotic conditions.
- Significant market potential, with the liver fibrosis treatment market expected to reach US$ 28.1 billion by 2026.
- None.
HOUSTON, Feb. 23, 2021 /PRNewswire/ -- Soliton, Inc., (Nasdaq: SOLY) ("Soliton" or the "Company"), a medical device company with a novel and proprietary aesthetic platform technology, today announced that a pre-clinical study in animals demonstrated positive results for the potential treatment of liver fibrosis. Validated laboratory and histological assessments in a mouse model demonstrated that Rapid Acoustic Pulse ("RAP") therapy reduced the effects of induced liver fibrosis 7-days following completion of carbon tetrachloride (CCL4) induction by
"We believe these results represent a significant discovery in our understanding of RAP's potential," commented Chris Capelli, Vice Chairman and CSO of Soliton. "Not only does this open up the possibility of addressing a significant unmet need in the treatment of liver fibrosis, these results help validate our mechanism of action with both cellulite and hypertrophic scars, as we believe RAP appears to target the fibroblast and reduce fibrosis in the tissue."
It is estimated that 6 to 7 percent of the world's population has liver fibrosis and that many who have it are unaware because they have no symptoms until it is too late for successful intervention. Coherent Market Insights Analysis indicates that the global liver fibrosis treatment market size is expected to witness a Compounded Annual Growth Rate of
"While we maintain a tight focus on our upcoming commercial launch of the RAP device in both cellulite reduction and tattoo removal, we believe exploring new indications with large markets and significant unmet needs, such as liver fibrosis, offers potential for increasing long-term shareholder value," said Brad Hauser, President and CEO of Soliton. "One of the things that distinguishes Soliton is the strength of the science that underlies RAP. This latest research underscores just how unique our acoustic shockwave technology is and how potentially important its applications could be. We look forward to continuing to examine the strong promise the RAP technology offers across many fibrotic conditions."
The RAP device is currently cleared by the FDA for temporary improvement in the appearance of cellulite. The RAP device is also indicated for use as an accessory to the 1064 nm Q-Switched laser for black ink tattoo removal in Fitzpatrick Skin Type I-III patients. The technology is not cleared for the treatment of liver fibrosis. The Company plans to begin selling the device for both tattoo removal and cellulite treatment in the first half of 2021.
Join our more than 200K subscribers here to follow the Company: https://soly-investors.com
About Soliton, Inc.
Soliton, Inc. is a medical device company with a novel and proprietary platform technology licensed from The University of Texas on behalf of MD Anderson Cancer Center. The Company's first FDA cleared commercial product will use rapid pulses of acoustic shockwaves as an accessory to lasers for the removal of unwanted tattoos and the treatment of cellulite. The Company is based in Houston, Texas, and is actively engaged in bringing the Rapid Acoustic Pulse ("RAP") device to the market. The Company believes this "Soliton" method has the potential to lower tattoo removal costs for patients, while increasing profitability to practitioners, compared to current laser removal methods. The Company also believe the technology will provide the first non-invasive acoustic technology to target the underlying causes of dimples and ridges in cellulite. Soliton is investigating potential additional capabilities of the RAP technology. The device is currently cleared in the United States only for use in tattoo removal and cellulite.
For more information about the Company, please visit: http://www.soliton.com
Forward-Looking Statements
This press release includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, which statements involve risks and uncertainties. Forward-looking statements in this press release include, without limitation, our ability to successfully replicate the results of this animal study in further clinical studies in either animals or humans and to launch our technology in the months to come. These statements relate to future events, future expectations, plans and prospects. Although Soliton believes that the expectations reflected in such forward-looking statements are reasonable as of the date made, actual results or outcomes may prove to be materially different from the expectations expressed or implied by such forward-looking statements. Soliton has attempted to identify forward-looking statements by terminology including ''believes,'' ''estimates,'' ''anticipates,'' ''expects,'' ''plans,'' ''projects,'' ''intends,'' ''potential,'' ''may,'' ''could,'' ''might,'' ''will,'' "would," ''should,'' ''approximately'' or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, including those discussed in our filings with the Securities and Exchange Commission ("SEC"), including under the heading " Risk Factors" in our most recently filed Form 10-K filed with the SEC and as updated in our Form 10-Q filings and in our other filings with the SEC. Any forward-looking statements contained in this release speak only as of its date. Soliton undertakes no obligation to update any forward-looking statements contained in this release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events.
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/soliton-announces-rap-treatment-reduces-effects-of-liver-fibrosis-by-42-in-animal-study-301232950.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/soliton-announces-rap-treatment-reduces-effects-of-liver-fibrosis-by-42-in-animal-study-301232950.html
SOURCE Soliton, Inc.
FAQ
What results did Soliton announce for RAP therapy on February 23, 2021?
What is the market potential for liver fibrosis treatment according to Soliton?
When does Soliton plan to launch its RAP device?