Silexion Therapeutics Reports Groundbreaking Positive Initial Data from Systemic Administration of SIL204 in Orthotopic Pancreatic Cancer Models
Silexion Therapeutics (NASDAQ: SLXN) announced positive initial data from orthotopic pancreatic cancer models for their SIL204 treatment. The study demonstrated that subcutaneously administered SIL204 effectively reduces both primary tumor growth and metastatic spread.
Key findings include:
- ~70% reduction in tumor cell numbers in AsPC-1 (KRAS G12D mutation) by day 28
- In Panc-1 (KRAS G12D), tumor cells decreased 12% while control group increased >100% by day 14
- ~80% reduction in BxPC-3 (KRAS wild-type) by day 28
- Significant reduction in metastatic spread across liver, intestine, spleen and stomach in Panc-1 and BxPC-3 models
The results validate SIL204's efficacy in a more clinically relevant setting, where human pancreatic tumor cells are implanted directly into the pancreas. The company is finalizing an updated development strategy based on these findings.
Silexion Therapeutics (NASDAQ: SLXN) ha annunciato dati iniziali positivi da modelli ortotopici di cancro pancreatico per il loro trattamento SIL204. Lo studio ha dimostrato che SIL204, somministrato per via sottocutanea, riduce efficacemente sia la crescita del tumore primario che la diffusione metastatica.
I risultati chiave includono:
- Riduzione di ~70% nel numero di cellule tumorali in AsPC-1 (mutazione KRAS G12D) entro il giorno 28
- In Panc-1 (KRAS G12D), le cellule tumorali sono diminuite del 12% mentre il gruppo di controllo è aumentato di oltre il 100% entro il giorno 14
- Riduzione di ~80% in BxPC-3 (KRAS wild-type) entro il giorno 28
- Riduzione significativa della diffusione metastatica nel fegato, intestino, milza e stomaco nei modelli Panc-1 e BxPC-3
I risultati convalidano l'efficacia di SIL204 in un contesto più clinicamente rilevante, dove le cellule tumorali pancreatiche umane vengono impiantate direttamente nel pancreas. L'azienda sta finalizzando una strategia di sviluppo aggiornata basata su questi risultati.
Silexion Therapeutics (NASDAQ: SLXN) anunció datos iniciales positivos de modelos ortotópicos de cáncer de páncreas para su tratamiento SIL204. El estudio demostró que SIL204 administrado por vía subcutánea reduce eficazmente tanto el crecimiento del tumor primario como la propagación metastásica.
Los hallazgos clave incluyen:
- Reducción de ~70% en el número de células tumorales en AsPC-1 (mutación KRAS G12D) para el día 28
- En Panc-1 (KRAS G12D), las células tumorales disminuyeron un 12% mientras que el grupo de control aumentó más del 100% para el día 14
- Reducción de ~80% en BxPC-3 (KRAS wild-type) para el día 28
- Reducción significativa en la propagación metastásica en el hígado, intestino, bazo y estómago en los modelos Panc-1 y BxPC-3
Los resultados validan la eficacia de SIL204 en un entorno más clínicamente relevante, donde las células tumorales pancreáticas humanas se implantan directamente en el páncreas. La empresa está finalizando una estrategia de desarrollo actualizada basada en estos hallazgos.
실렉시온 테라퓨틱스 (NASDAQ: SLXN)는 SIL204 치료를 위한 정위치 췌장암 모델에서 긍정적인 초기 데이터를 발표했습니다. 이 연구는 피하 주사된 SIL204가 1차 종양 성장과 전이 확산을 효과적으로 감소시킨다는 것을 보여주었습니다.
주요 발견 사항은 다음과 같습니다:
- AsPC-1 (KRAS G12D 변이)에서 28일째 종양 세포 수가 약 70% 감소
- Panc-1 (KRAS G12D)에서 종양 세포가 12% 감소한 반면, 대조군은 14일째 100% 이상 증가
- BxPC-3 (KRAS 정상형)에서 28일째 약 80% 감소
- Panc-1 및 BxPC-3 모델에서 간, 장, 비장 및 위로의 전이 확산이 상당히 감소
이 결과는 인간 췌장 종양 세포가 췌장에 직접 이식되는 보다 임상적으로 관련된 환경에서 SIL204의 효능을 검증합니다. 회사는 이러한 발견을 바탕으로 업데이트된 개발 전략을 마무리하고 있습니다.
Silexion Therapeutics (NASDAQ: SLXN) a annoncé des données initiales positives issues de modèles orthotopiques de cancer du pancréas pour leur traitement SIL204. L'étude a démontré que SIL204 administré par voie sous-cutanée réduit efficacement à la fois la croissance de la tumeur primaire et la propagation métastatique.
Les résultats clés comprennent:
- Réduction d'environ 70% du nombre de cellules tumorales dans AsPC-1 (mutation KRAS G12D) au jour 28
- Dans Panc-1 (KRAS G12D), les cellules tumorales ont diminué de 12% tandis que le groupe de contrôle a augmenté de plus de 100% au jour 14
- Réduction d'environ 80% dans BxPC-3 (KRAS wild-type) au jour 28
- Réduction significative de la propagation métastatique dans le foie, l'intestin, la rate et l'estomac dans les modèles Panc-1 et BxPC-3
Les résultats valident l'efficacité de SIL204 dans un contexte cliniquement plus pertinent, où les cellules tumorales pancréatiques humaines sont implantées directement dans le pancréas. L'entreprise finalise une stratégie de développement mise à jour basée sur ces résultats.
Silexion Therapeutics (NASDAQ: SLXN) hat positive erste Daten aus orthotopischen Modellen von Bauchspeicheldrüsenkrebs für ihre Behandlung SIL204 bekannt gegeben. Die Studie zeigte, dass subkutan verabreichtes SIL204 sowohl das Wachstum des Primärtumors als auch die metastatische Ausbreitung effektiv reduziert.
Wichtige Ergebnisse sind:
- ~70% Reduktion der Tumorzellzahlen in AsPC-1 (KRAS G12D Mutation) bis Tag 28
- In Panc-1 (KRAS G12D) verringerten sich die Tumorzellen um 12%, während die Kontrollgruppe bis Tag 14 um über 100% zunahm
- ~80% Reduktion in BxPC-3 (KRAS Wildtyp) bis Tag 28
- Signifikante Reduktion der metastatischen Ausbreitung in Leber, Darm, Milz und Magen in den Modellen Panc-1 und BxPC-3
Die Ergebnisse validieren die Wirksamkeit von SIL204 in einem klinisch relevanteren Setting, in dem menschliche Bauchspeicheldrüsenzellen direkt in die Bauchspeicheldrüse implantiert werden. Das Unternehmen finalisiert eine aktualisierte Entwicklungsstrategie basierend auf diesen Erkenntnissen.
- Strong efficacy data: 70-80% tumor reduction in multiple cancer cell lines
- First demonstration of metastasis reduction across multiple organs
- Successful systemic delivery via subcutaneous administration
- Effective against both KRAS mutant and wild-type tumors
- Still in preclinical stage, requiring further clinical validation
- Results to animal models, human efficacy yet to be demonstrated
Insights
Silexion's new data represents a significant technical advancement in their RNAi therapeutic approach for pancreatic cancer. The transition from subcutaneous xenograft models to orthotopic models – where tumor cells grow in their native pancreatic environment – is a important step forward in preclinical validation.
The efficacy demonstrated across multiple cell lines with different KRAS mutation profiles (70-80% reduction in tumor burden) is particularly promising. KRAS mutations, especially G12D, are notorious drivers in pancreatic cancer with targeted treatment options. What's most impressive is SIL204's ability to reduce metastatic spread while being administered subcutaneously – addressing two major challenges in pancreatic cancer treatment: metastatic disease and delivery method.
Pancreatic cancer's poor prognosis stems largely from its tendency to metastasize early, often before diagnosis. A therapy that can simultaneously target primary tumors and metastases through minimally invasive administration would be revolutionary if these results translate to humans.
However, these remain preclinical results – the path from orthotopic models to human efficacy is still substantial. The company appears to be planning their clinical strategy, but investors should understand that years of clinical development likely remain before potential commercialization. The efficacy signals are encouraging, but human trials will need to confirm both safety and efficacy in this difficult-to-treat cancer type.
These preclinical results potentially strengthen Silexion's competitive position in the KRAS-targeting therapeutic landscape. With a market cap of $8.8 million and significant progress in their lead candidate, the company appears undervalued compared to other early-stage oncology biotechs targeting similar pathways.
RNAi approaches for KRAS-driven cancers represent a differentiated strategy in a field where many companies pursue small molecule inhibitors with mixed results. The systemic delivery validation is particularly valuable – addressing the long-standing challenge of RNA therapeutic delivery to solid tumors.
Notably, SIL204 showed efficacy in both KRAS-mutant and wild-type models, suggesting potential broader application beyond just KRAS-mutated cancers. This could significantly expand the addressable patient population if confirmed in clinical studies.
The company's announcement of an upcoming development strategy update signals potential acceleration in their timeline. For investors, key upcoming catalysts will likely include IND-enabling studies, regulatory interactions, and early clinical trial designs. The pancreatic cancer market, while smaller than some other oncology indications, represents a high unmet need with competition and potential for premium pricing.
While encouraging, these results should be viewed in context – many promising preclinical cancer therapies fail in human testing. The company will need substantial additional funding to advance through clinical trials, likely resulting in dilution for current shareholders. The path to market remains long, but these data strengthen Silexion's scientific foundation.
Silexion Therapeutics' Latest Data Demonstrates that Subcutaneously Administered SIL204 Reduces Both Primary Tumors and Metastases in Clinically Relevant Orthotopic Models Where Human Pancreatic Cancer Cells Grow in Their Native Environment
Grand Cayman, Cayman Islands, March 05, 2025 (GLOBE NEWSWIRE) -- Silexion Therapeutics Corp. (NASDAQ: SLXN) ("Silexion" or the "Company"), a clinical-stage biotechnology company pioneering RNA interference (RNAi) therapies for KRAS-driven cancers, today announced positive data from orthotopic pancreatic cancer models demonstrating that subcutaneously administered SIL204 effectively reduces both primary tumor growth and metastatic spread.
These findings represent a significant advancement over previously reported data by providing further validation of SIL204's efficacy in the more clinically relevant orthotopic setting, where human pancreatic tumor cells are implanted directly into the pancreas to better mimic human disease progression, including, for the first time, in metastasis patterns.
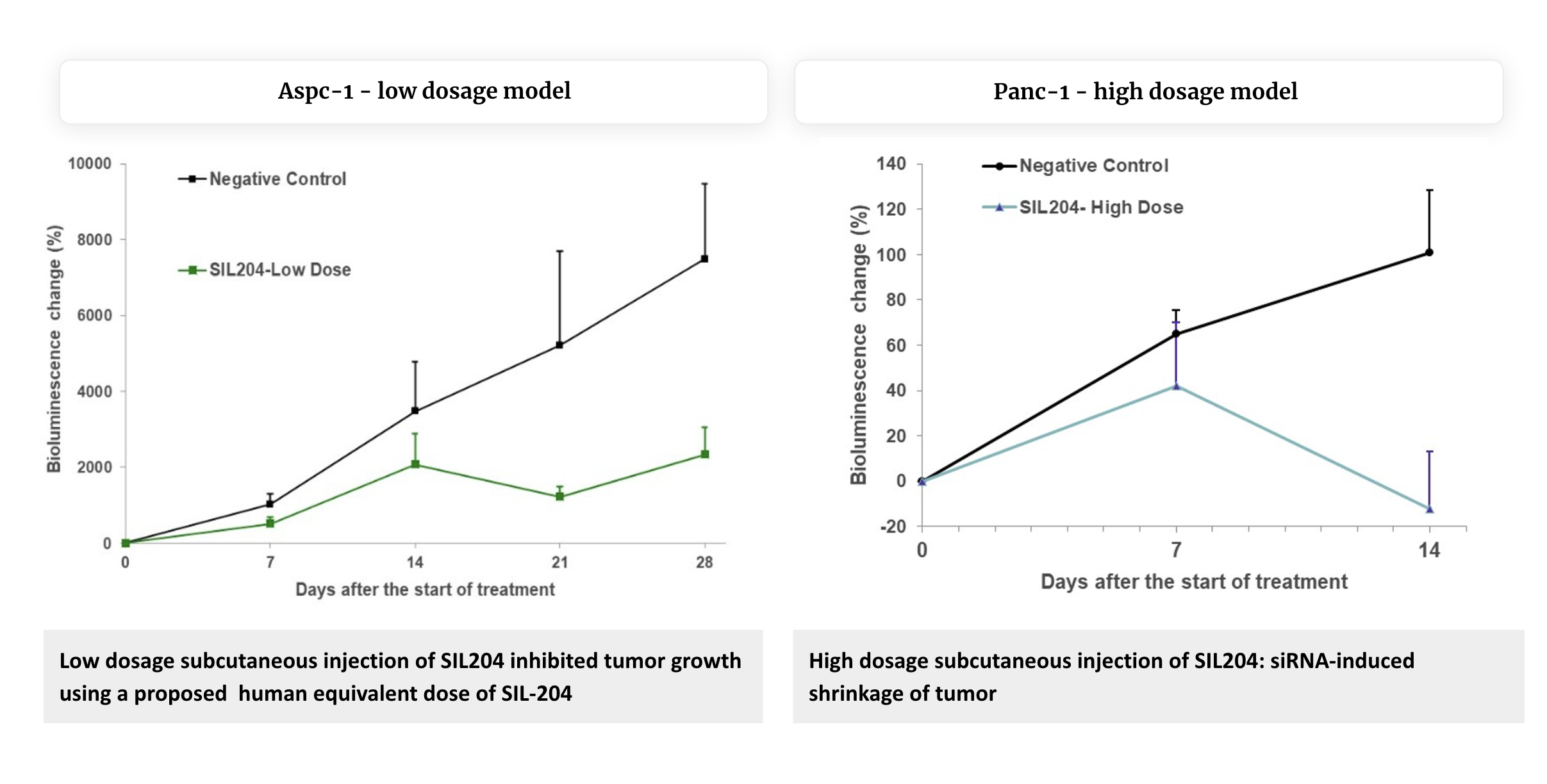
Key New Findings
- Initial validation using orthotopic models: SIL204 administered subcutaneously (systemically) showed significant efficacy in orthotopic xenograft models where tumors grow in their native pancreatic environment, representing a more clinically relevant setting than our previous subcutaneous xenograft models.
- Cell line-specific efficacy profiles: SIL204 showed robust activity across multiple pancreatic cancer cell lines with different KRAS mutation profiles:
- In AsPC-1 (harboring KRAS G12D mutation): ~
70% reduction in overall bioluminescence (an indication of tumor cell number) as compared to the control group, by day 28. - In Panc-1 (harbouring KRAS G12D mutation): Bioluminescence or tumor cell numbers decreased dramatically in a dose-dependent manner, with the highest-dose group showing the most significant effect. Bioluminescence in the control group increased by approximately more than
100% while in the SIL204 treated group it decreased by12% compared to baseline, a substantial reduction by day 14 compared with the control group (P-value < 0.05), demonstrating the significant therapeutic impact of SIL204. - In BxPC-3 (additional KRAS wild-type model): ~
80% reduction in overall bioluminescence, as compared to the control group, by day 28.
- In AsPC-1 (harboring KRAS G12D mutation): ~
- Metastasis reduction demonstrated for the first time: SIL204 treatment significantly reduced metastatic spread to secondary organs; substantially lowering metastatic burden across the liver, intestine, spleen and stomach in the two models checked for the various organs, Panc-1 and BxPC-3 models.
- Initial validation for systemic delivery efficacy: Subcutaneous administration of SIL204 proved effective in reaching and treating pancreatic tumors and their metastases, confirming systemic delivery as a viable administration route.
"These orthotopic model results represent a pivotal advancement in our development program," said Mitchell Shirvan, Ph.D., CSO of Silexion. "While our previous data showed SIL204's ability to reduce tumor growth in standard models, these new findings provide initial validation of its potential effectiveness in a much more clinically relevant setting. Particularly exciting is the demonstration that SIL204 can significantly reduce metastatic spread when administered subcutaneously, suggesting potential for treating both primary and metastatic disease with a minimally-invasive delivery method."
Based on these encouraging results, Silexion is actively exploring an expanded development plan for SIL204 using the systemic administration approach. The Company is finalizing an updated development strategy that leverages these new findings and expects to provide more detailed information in the coming weeks, as previously indicated in recent announcements.
"These results mark the first time we've demonstrated SIL204's ability to address metastatic disease through subcutaneous administration," added Ilan Hadar, Chairman and CEO of Silexion. "The ability to deliver our therapy systemically and still effectively target both primary pancreatic tumors and their metastases represents a significant potential advantage for treating this devastating disease."
About Silexion Therapeutics
Silexion Therapeutics is a pioneering clinical-stage, oncology-focused biotechnology company developing innovative RNA interference (RNAi) therapies to treat solid tumors driven by KRAS mutations, the most common oncogenic driver in human cancers. The company's first-generation product, LODER™, has shown promising results in a Phase 2 clinical trial for non-resectable pancreatic cancer. Silexion is also advancing its next-generation siRNA candidate, SIL204, designed to target a broader range of KRAS mutations and showing significant potential in preclinical studies. The company remains committed to pushing the boundaries of therapeutic innovation in oncology, with a focus on improving outcomes for patients with difficult-to-treat cancers. For more information please visit: https://silexion.com
Notice Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the federal securities laws. All statements other than statements of historical fact contained in this communication, including statements regarding Silexion’s business strategy and ongoing studies are forward-looking statements. These forward-looking statements are generally identified by terminology such as "may", "should", "could", "might", "plan", "possible", "project", "strive", "budget", "forecast", "expect", "intend", "will", "estimate", "anticipate", "believe", "predict", "potential" or "continue", or the negatives of these terms or variations of them or similar terminology. Forward-looking statements involve a number of risks, uncertainties, and assumptions, and actual results or events may differ materially from those projected or implied in those statements. Important factors that could cause such differences include, but are not limited to: (i) Silexion’s ability to successfully complete preclinical studies and initiate clinical trials; (ii) Silexion’s strategy, future operations, financial position, projected costs, prospects, and plans; (iii) the impact of the regulatory environment and compliance complexities; (iv) expectations regarding future partnerships or other relationships with third parties; (v) Silexion’s future capital requirements and sources and uses of cash, including its ability to obtain additional capital; and (vi) other risks and uncertainties set forth in the documents filed or to be filed with the SEC by the company, including the registration statement on Form S-1 filed with the SEC on February 12, 2025. Silexion cautions you against placing undue reliance on forward-looking statements, which reflect current beliefs and are based on information currently available as of the date a forward-looking statement is made. Forward-looking statements set forth herein speak only as of the date they are made. Silexion undertakes no obligation to revise forward-looking statements to reflect future events, changes in circumstances, or changes in beliefs, except as otherwise required by law.
Company Contact
Silexion Therapeutics Corp
Ms. Mirit Horenshtein Hadar, CFO
mirit@silexion.com
Capital Markets & IR Contact
Arx | Capital Markets Advisors
North American Equities Desk
silexion@arxadvisory.com








