Computer-guided surgery with Smith+Nephew's hip replacement implants shown to significantly improve survivorship and increase patient satisfaction compared to conventional surgery
Smith+Nephew (LSE: SN, NYSE: SNN) announces a study demonstrating that its computer-guided technology for total hip arthroplasty (THA), known as RI.HIP NAVIGATION, significantly lowers revision rates and enhances patient satisfaction. Utilizing the National Joint Registry of England, Wales, and Northern Ireland, the study found a 55% lower risk of revision at ten years (1.06% vs. 3.88% for conventional THA). Higher patient satisfaction rates were also reported in the computer-guided group. This technology aims to improve long-term outcomes for patients undergoing hip replacement.
- Significantly lower revision rate at 10 years with computer-guided THA (1.06%) compared to conventional THA (3.88%; p=0.005).
- 55% lower risk of revision at 10 years with computer-guided THA (p=0.038).
- Increased patient satisfaction in the computer-guided group compared to conventional THA (p=0.003).
- None.
Insights
Analyzing...
LONDON, April 28, 2021 /PRNewswire/ -- Smith+Nephew (LSE: SN, NYSE: SNN), the global medical technology business, today announces a new study showing that computer-guided technology for total hip arthroplasty (THA) – such as Smith+Nephew's RI.HIP NAVIGATION – significantly reduces the risk of revision and increases patient satisfaction when using Smith+Nephew implants.
The study is the first of its kind using the dataset from the world's largest arthroplasty register (National Joint Registry of England, Wales and Northern Ireland) to investigate the effect of computer-guided THA surgery on implant survivorship.1 The data reported on THA surgery performed using Smith+Nephew hip replacement components implanted for osteoarthritis since 2003. Presented at the 2021 World Arthroplasty Congress (WAC), the results demonstrated:
- Significantly lower revision rate at 10 years with computer-guided (
1.06% ) vs. conventional THA (3.88% ; p=0.005) 55% lower risk of revision at 10 years with computer-guided vs. conventional THA (p=0.038)- Patient satisfaction was significantly higher in the computer-guided group compared to conventional THA (p=0.003)
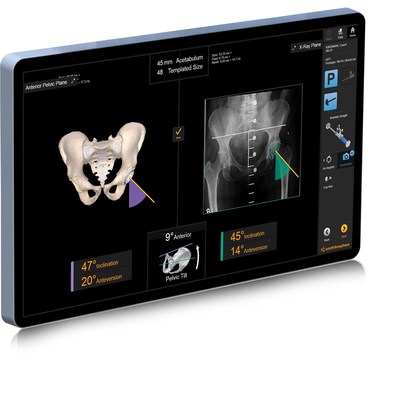
"Computer-guided technology for total hip arthroplasty has been around for more than twenty years and this study confirms that it greatly improves a patient's chance for long-term success and their overall satisfaction," said Prof. Edward T. Davis FRCS, of The Royal Orthopaedic Hospital NHS Foundation Trust. "RI.HIP NAVIGATION helps me take control of individual patient pelvic tilt, leg length and offset measurement while assisting with cup placement by giving a predicted view of the post-op AP X-ray in surgery. This gives me supreme confidence that I'm performing the most accurate THA procedure for my patient and ensuring the best possible result."
RI.HIP NAVIGATION technology is designed to help maximise accuracy and reproducibility by delivering patient-specific component alignment – a critical factor for surgeons when assessing individual THA cases. Using this technology with Smith+Nephew's leading POLAR3® Primary Total Hip System – comprised of POLARSTEM®, OXINIUM® and R3® acetabular components – has the potential to take THA to the next level of performance for surgeons and patients.
"Conventional total hip arthroplasty is considered to be a very successful procedure, however there is always room for improvement," said Randy Kilburn, Senior Vice President of Commercial Marketing, Orthopaedics for Smith+Nephew. "RI.HIP NAVIGATION combined with our best-in-class POLAR3® THA construct may considerably improve implant survivorship and help patients return to living their Life Unlimited."
To learn more about RI.HIP NAVIGATION and Smith+Nephew's Real Intelligence brand of enabling technology solutions designed to address clinical challenges through the continuum of care, please visit www.real-intelligence.com.
Reference
1. Davis ET, McKinney KD, Kamali A, Kuljaca S, Pagkalos J. Computer guided total hip arthroplasty is associated with a reduced risk of revision and increased patient satisfaction. An analysis of a single manufacturer acetabular components from the National Joint Registry of England, Wales, Northern Ireland and the Isle of Man. Poster presented at: World Arthroplasty Congress Virtual Meeting; April 22-24, 2021.
About Smith+Nephew
Smith+Nephew is a portfolio medical technology business that exists to restore people's bodies and their self-belief by using technology to take the limits off living. We call this purpose 'Life Unlimited'. Our 18,000 employees deliver this mission every day, making a difference to patients' lives through the excellence of our product portfolio, and the invention and application of new technologies across our three global franchises of Orthopaedics, Advanced Wound Management and Sports Medicine & ENT.
Founded in Hull, UK, in 1856, we now operate in more than 100 countries, and generated annual sales of
For more information about Smith+Nephew, please visit www.smith-nephew.com and follow us on Twitter, LinkedIn, Instagram or Facebook.
Forward-looking Statements
This document may contain forward-looking statements that may or may not prove accurate. For example, statements regarding expected revenue growth and trading margins, market trends and our product pipeline are forward-looking statements. Phrases such as "aim", "plan", "intend", "anticipate", "well-placed", "believe", "estimate", "expect", "target", "consider" and similar expressions are generally intended to identify forward-looking statements. Forward-looking statements involve known and unknown risks, uncertainties and other important factors that could cause actual results to differ materially from what is expressed or implied by the statements. For Smith+Nephew, these factors include: risks related to the impact of COVID-19, such as the depth and longevity of its impact, government actions and other restrictive measures taken in response, material delays and cancellations of elective procedures, reduced procedure capacity at medical facilities, restricted access for sales representatives to medical facilities, or our ability to execute business continuity plans as a result of COVID-19; economic and financial conditions in the markets we serve, especially those affecting health care providers, payers and customers (including, without limitation, as a result of COVID-19); price levels for established and innovative medical devices; developments in medical technology; regulatory approvals, reimbursement decisions or other government actions; product defects or recalls or other problems with quality management systems or failure to comply with related regulations; litigation relating to patent or other claims; legal compliance risks and related investigative, remedial or enforcement actions; disruption to our supply chain or operations or those of our suppliers (including, without limitation, as a result of COVID-19); competition for qualified personnel; strategic actions, including acquisitions and dispositions, our success in performing due diligence, valuing and integrating acquired businesses; disruption that may result from transactions or other changes we make in our business plans or organisation to adapt to market developments; and numerous other matters that affect us or our markets, including those of a political, economic, business, competitive or reputational nature. Please refer to the documents that Smith+Nephew has filed with the U.S. Securities and Exchange Commission under the U.S. Securities Exchange Act of 1934, as amended, including Smith+Nephew's most recent annual report on Form 20-F, for a discussion of certain of these factors. Any forward-looking statement is based on information available to Smith+Nephew as of the date of the statement. All written or oral forward-looking statements attributable to Smith+Nephew are qualified by this caution. Smith+Nephew does not undertake any obligation to update or revise any forward-looking statement to reflect any change in circumstances or in Smith+Nephew's expectations.
® Trademark of Smith+Nephew. Certain marks registered US Patent and Trademark Office.
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/computer-guided-surgery-with-smithnephews-hip-replacement-implants-shown-to-significantly-improve-survivorship-and-increase-patient-satisfaction-compared-to-conventional-surgery-301278677.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/computer-guided-surgery-with-smithnephews-hip-replacement-implants-shown-to-significantly-improve-survivorship-and-increase-patient-satisfaction-compared-to-conventional-surgery-301278677.html
SOURCE Smith & Nephew plc