Revelation Biosciences Inc. Announces Preclinical Biomarker Data Supporting Activity of REVTx-300
Revelation Biosciences Inc. (NASDAQ: REVB) reported statistically significant preclinical results for REVTx-300, a potential therapy for acute and chronic kidney disease. In a validated model, treatment with REVTx-300 led to significant reductions in TGF-β and increases in anti-inflammatory markers IL-10, hepcidin, and NGAL. No significant inflammation markers increased, indicating a potential reduction in fibrosis. The company plans to publish these findings in 2023 and is preparing to initiate clinical studies. Positive results were previously reported on October 25, 2022, and November 18, 2022, regarding renal fibrosis reduction.
- Statistically significant reduction in TGF-β levels and increased IL-10, hepcidin, and NGAL after treatment with REVTx-300.
- Demonstrated potential for REVTx-300 to reduce fibrosis in a validated preclinical kidney disease model.
- None.
Insights
Analyzing...
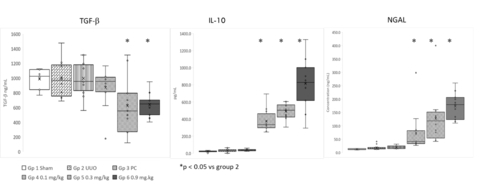
(Graphic: Business Wire)
In this validated preclinical model, administration of REVTx-300 caused significant reduction in circulating transforming growth factor-β (TGF-β) in a dose dependent manner relative to the positive control group. In addition, REVTx-300 significantly increased circulating anti-inflammatory interleukin-10 (IL-10), hepcidin, and neutrophil gelatinase-associated lipocalin (NGAL) in all groups in a dose dependent manner, relative to the positive control group (the unilateral ureteral obstruction (UUO) model, described below).
TGF-β is a key driver of fibrogenesis and contributes directly to the deposition of collagen through excessive production of extracellular matrix. IL-10 is characterized as an anti-inflammatory cytokine, due to its capability to reduce the generation of pro-inflammatory mediators. Hepcidin and NGAL sequester iron to prevent iron-mediated reactive oxygen tissue damage.
There were no significant increases in markers of inflammation. These results provide mechanistic evidence for the reduction in fibrosis observed in the UUO model in response to treatment with REVTx-300. Revelation plans to seek publication of the full results during 2023.
Revelation originally released positive results (
“Redirection of the inflammatory response has tremendous potential to treat a wide variety of diseases associated with fibrosis,” said
Fibrosis, or the formation of scar tissue, is the final, common pathway of many diseases which can have devastating effects, including acute kidney injury, chronic kidney injury, nonalcoholic steatohepatitis, myocarditis, and cancer. Revelation intends to further investigate the potential anti-inflammatory and anti-fibrotic benefit of PHAD, including its effect on NLRP3-mediated inflammasome activation in animal models of AKI, CKD, nonalcoholic steatohepatitis (NASH), and cancer.
About REVTx-300 Preclinical Study
The unilateral ureteral obstruction (UUO) model is appropriate for studying the anti-inflammatory and anti-fibrotic effects of potential new therapies for acute and chronic kidney disease as complete ureteral obstruction of one kidney results in significant inflammation and subsequent fibrosis of the affected kidney over a 7-day period.
The present study consisted of 6 groups with the following outcomes on renal cortical fibrosis as measured by detection of collagen deposition using picosirius red stained histology sections assessed at three different sampling depths.
-
Group 1 animals had surgery with no UUO (Sham) and received vehicle only (collagen deposition: 2.36 ±
0.44% ). -
Group 2 animals had UUO surgery and received vehicle only (collagen deposition: 4.88 ±
0.51% ). -
Group 3 animals had UUO surgery and received SB-525334, a known TGF-β inhibitor of fibrosis (new collagen deposition: 3.02 ±
0.37% ,75% reduction vs Group 2 - Sham, p < 0.05). -
Group 4 animals had UUO surgery and received 0.1 mg/kg REVTx-300 (new collagen deposition: 4.96 ± 0.95 %,
1% reduction vs Group 2 – Sham). -
Group 5 animals had UUO surgery and received 0.3 mg/kg REVTx-300 (new collagen deposition: 3.82 ±
0.91% ,42% reduction vs Group 2 – Sham, p < 0.05). -
Group 6 animals had UUO surgery and received 0.9 mg/kg REVTx-300 (new collagen deposition: 3.45 ±
0.54% ,57% reduction vs Group 2 – Sham, p < 0.05).
About REVTx-300
REVTx-300 is a proprietary formulation for systemic administration of PHAD and is being developed as a potential therapy for the treatment of acute and chronic organ disease including chronic kidney disease (CKD), acute kidney injury (AKI), myocarditis, and nonalcoholic steatohepatitis (NASH). Chronic disease of an organ, due to chronic inflammation and subsequent fibrosis, follows a pattern of perpetual and ongoing destruction of living functional cells and subsequent replacement by the non-functional protein, collagen, resulting in fibrosis (scar tissue) (Wilson). The establishment of fibrosis and subsequent death of the organ is driven by ongoing inflammatory processes and reactive oxygen species associated with the innate immune response. Revelation believes that redirection of the innate immune response with REVTx-300 from a pro-inflammatory state to an anti-inflammatory (protective) state may rebalance the innate immune response to slow down or halt the progressive destruction and scarring of organ tissue, allowing the healing process to take place. Revelation plans to initiate clinical studies in 2023.
About
Forward-Looking Statements
This press release contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts. These forward-looking statements are generally identified by the words "anticipate", "believe", "expect", "estimate", "plan", "outlook", and "project" and other similar expressions. We caution investors that forward-looking statements are based on management’s expectations and are only predictions or statements of current expectations and involve known and unknown risks, uncertainties and other factors that may cause actual results to be materially different from those anticipated by the forward-looking statements. Revelation cautions readers not to place undue reliance on any such forward looking statements, which speak only as of the date they were made. The following factors, among others, could cause actual results to differ materially from those described in these forward-looking statements: the ability of Revelation to meet its financial and strategic goals, due to, among other things, competition; the ability of Revelation to grow and manage growth profitability and retain its key employees; the possibility that the Revelation may be adversely affected by other economic, business, and/or competitive factors; risks relating to the successful development of Revelation’s product candidates; the clinical utility of an increase in intranasal cytokine levels as a biomarker of viral infections; the ability to successfully complete planned clinical studies of its product candidates; the risk that we may not fully enroll our clinical studies or enrollment will take longer than expected; risks relating to the occurrence of adverse safety events and/or unexpected concerns that may arise from data or analysis from our clinical studies; changes in applicable laws or regulations; expected initiation of the clinical studies, the timing of clinical data; the outcome of the clinical data, including whether the results of such study is positive or whether it can be replicated; the outcome of data collected, including whether the results of such data and/or correlation can be replicated; the timing, costs, conduct and outcome of our other clinical studies; the anticipated treatment of future clinical data by the FDA, the EMA or other regulatory authorities, including whether such data will be sufficient for approval; the success of future development activities for its product candidates; potential indications for which product candidates may be developed; the potential impact that COVID‑19 may have on Revelation’s suppliers, vendors, regulatory agencies, employees and the global economy as a whole; the ability of Revelation to maintain the listing of its securities on NASDAQ; investor sentiment relating to SPAC related going public transactions; the expected duration over which Revelation’s balances will fund its operations; and other risks and uncertainties described herein, as well as those risks and uncertainties discussed from time to time in other reports and other public filings with the
View source version on businesswire.com: https://www.businesswire.com/news/home/20230207005660/en/
Company Contacts
Sandra Vedrick
Vice President, Investor Relations & Human Resources
Email: svedrick@revbiosciences.com
and
Chief Financial Officer
Email: czygmont@revbiosciences.com
Source:







