Gemini Priming Attenuates Inflammation in Human Peripheral Blood Mononuclear Cells
– Observed response is excellent surrogate of the potential of Gemini in target indications –
– Equivalent response anticipated in Phase 1b study CKD patients –
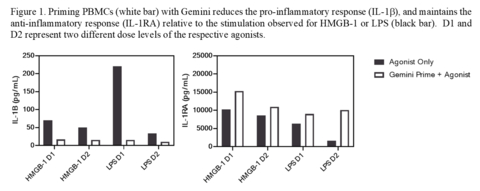
In this study, human PBMCs were primed with either Gemini or placebo followed by challenge with clinically relevant promoter molecules of inflammation including high mobility box protein-1 (HMGB-1) and lipopolysaccharide (LPS) in vitro. HMGB-1 is an endogenous damage associated molecular pattern (DAMP) that is generated during tissue injury (e.g. during surgery) which drives the inflammatory response. LPS is an exogenous pathogen associated molecular pattern (PAMP) that is generated from a bacterial infection and drives the inflammatory response associated with infection (e.g. fever).
After exposure of Gemini-primed PMBCs to either HMGB-1 or LPS, levels of multiple proinflammatory and anti-inflammatory cytokines including IL-1 beta, TNF-alpha, IL-6, IL-1 Receptor Antagonist, and IL-10 were measured. Priming with Gemini significantly decreased proinflammatory cytokines and significantly increased anti-inflammatory cytokines, relative to placebo. Figure 1 shows two relevant measures following HMGB-1 and LPS challenge. This fundamental change in responsiveness at the cellular level demonstrates how Gemini will protect against inflammation-mediated damage. Revelation intends to share the full results from this study in a future publication.
As a part of the PRIME (PReconditioning IMmunostimulatory Evaluation) Phase 1b clinical study in patients with Stage 3 and 4 Chronic Kidney Disease (CKD), Revelation is collecting PBMCs from dosed patients and anticipates demonstration of the attenuated response to HMGB-1 and LPS ex vivo relative to placebo subjects. Top-line data comprising safety, tolerability, and this demonstration of protection from relevant promotors of inflammation at a cellular level are expected by mid 2025. Data from the PRIME clinical study will support future development of the GEM-CKD, GEM-AKI and GEM-PSI programs.
“These exciting results further demonstrate the game-changing potential of Gemini to attenuate the inflammatory response to clinically relevant DAMPS and PAMPS,” said James Rolke, Chief Executive Officer of Revelation. “We look forward to demonstrating the same protective effect in cells collected from CKD patients, which will be an excellent surrogate of future efficacy of Gemini in our target indications.”
About AKI
AKI, also known as acute renal failure, is defined as a rapid loss of kidney function. AKI causes a build-up of waste products in blood and makes it more difficult for kidneys to maintain the correct balance of fluid in the body. AKI can also have a significant impact on other organs such as the brain, heart, and lungs.
About CKD
Chronic kidney disease is a pervasive problem in
About Gemini
Gemini is an intravenously administrated, proprietary formulation of phosphorylated hexaacyl disaccharide (PHAD®) that reduces the damage associated with inflammation by reprogramming the innate immune system to respond to stress (trauma, infection, etc.) in an attenuated manner. Revelation has conducted multiple preclinical studies demonstrating the therapeutic potential of Gemini in the target indications. Revelation previously announced positive Phase 1 clinical data for intravenous treatment with Gemini. The primary safety endpoint was met in the Phase 1 study, and results demonstrated statistically significant pharmacodynamic activity as observed through expected changes in multiple biomarkers including upregulation of IL-10.
Gemini is being developed for multiple indications including as a pretreatment to prevent or reduce the severity and duration of acute kidney injury (GEMINI-AKI program), and as pretreatment to prevent or reduce the severity and duration of post-surgical infection (GEMINI-PSI program). In addition, Gemini may be a treatment to stop or slow the progression of chronic kidney disease (GEMINI-CKD program).
About Revelation Biosciences, Inc.
Revelation Biosciences, Inc. is a clinical stage life sciences company focused on harnessing the power of trained immunity for the prevention and treatment of disease using its proprietary formulation Gemini. Revelation has multiple ongoing programs to evaluate Gemini, including as a prevention for post-surgical infection, as a prevention for acute kidney injury, and for the treatment of chronic kidney disease.
For more information on Revelation, please visit www.RevBiosciences.com.
Forward-Looking Statements
This press release contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts. These forward-looking statements are generally identified by the words "anticipate", "believe", "expect", "estimate", "plan", "outlook", and "project" and other similar expressions. We caution investors that forward-looking statements are based on management’s expectations and are only predictions or statements of current expectations and involve known and unknown risks, uncertainties and other factors that may cause actual results to be materially different from those anticipated by the forward-looking statements. Revelation cautions readers not to place undue reliance on any such forward looking statements, which speak only as of the date they were made. The following factors, among others, could cause actual results to differ materially from those described in these forward-looking statements: the ability of Revelation to meet its financial and strategic goals, due to, among other things, competition; the ability of Revelation to grow and manage growth profitability and retain its key employees; the possibility that the Revelation may be adversely affected by other economic, business, and/or competitive factors; risks relating to the successful development of Revelation’s product candidates; the ability to successfully complete planned clinical studies of its product candidates; the risk that we may not fully enroll our clinical studies or enrollment will take longer than expected; risks relating to the occurrence of adverse safety events and/or unexpected concerns that may arise from data or analysis from our clinical studies; changes in applicable laws or regulations; expected initiation of the clinical studies, the timing of clinical data; the outcome of the clinical data, including whether the results of such study is positive or whether it can be replicated; the outcome of data collected, including whether the results of such data and/or correlation can be replicated; the timing, costs, conduct and outcome of our other clinical studies; the anticipated treatment of future clinical data by the FDA, the EMA or other regulatory authorities, including whether such data will be sufficient for approval; the success of future development activities for its product candidates; potential indications for which product candidates may be developed; the ability of Revelation to maintain the listing of its securities on NASDAQ; the expected duration over which Revelation’s balances will fund its operations; and other risks and uncertainties described herein, as well as those risks and uncertainties discussed from time to time in other reports and other public filings with the SEC by Revelation.
View source version on businesswire.com: https://www.businesswire.com/news/home/20250317682472/en/
Company Contacts
Mike Porter
Investor Relations
Porter LeVay & Rose Inc.
Email: mike@plrinvest.com
Chester Zygmont, III
Chief Financial Officer
Revelation Biosciences Inc.
Email: czygmont@revbiosciences.com
Source: Revelation Biosciences, Inc.







