Revelation Biosciences Announces Phase 1 Clinical Study of Gemini Met the Primary Safety Endpoint and Showed Statistically Significant Biomarker Activity
Revelation Biosciences announced that its Phase 1 clinical study of Gemini met its primary safety endpoint and showed statistically significant biomarker activity. The study, conducted with 40 healthy volunteers aged 18-55 in Australia, found that Gemini was safe and well-tolerated at pharmacologically active doses. Significant dose-dependent changes in key biomarkers, such as interleukin-1RA, neutrophil gelatinase lipocalin, C-reactive protein, and IL-6, were observed, indicating the drug's immunostimulatory effects. The Phase 1 results support further development across multiple indications, including acute kidney injury prevention and postoperative infection prevention. The maximum tolerated dose identified will guide the upcoming Phase 1b study in chronic kidney disease patients planned for late 2024.
- Phase 1 clinical study met the primary safety endpoint.
- Statistically significant dose-dependent changes in key biomarkers observed.
- Gemini was well-tolerated at pharmacologically active doses.
- No significant increases in serum TNF-α and IL-1β, indicating lower pro-inflammatory activity.
- Supports further development across multiple indications like AKI and postoperative infection prevention.
- Maximum tolerated dose identified for Phase 1b study.
- Adverse events such as transient headache, chills, body aches, and vomiting were observed.
- No significant upregulation of IL-10, which is important for anti-inflammatory responses.
Insights
Revelation Biosciences' announcement of the Phase 1 clinical study results for its drug, Gemini, is a significant milestone in the realm of immunotherapy. The study met its primary safety endpoint, indicating that Gemini was well tolerated across different doses and the administration led to a dose-dependent increase in key biomarkers. This is important for progressing to the next phase of clinical trials.
From a medical research perspective, the most notable aspect is the statistically significant changes in biomarkers like interleukin-1RA (IL-1RA), neutrophil gelatinase lipocalin (NGAL) and c-reactive protein (CRP). These indicators are known to play significant roles in immune response and inflammation resolution. The absence of significant increases in TNF-α and IL-1β is particularly promising as it suggests a targeted immune modulation without triggering excessive inflammation.
The data indicates potential clinical efficacy in preventing acute kidney injury (AKI) and post-surgical infections, aligning with preclinical expectations. This consistency across preclinical and clinical data enhances the credibility of the drug’s effectiveness. The well-tolerated nature of the drug, with primarily mild adverse events, further supports its potential for broader application in human trials.
In summary, this study provides a solid foundation for future clinical investigations, potentially opening doors for new treatment avenues in immunotherapy and inflammation-related diseases.
From a financial perspective, Revelation Biosciences' positive Phase 1 results for Gemini could be a game-changer. Successful early-stage trials often lead to increased investor confidence and can drive up stock prices. The company's progress in safety and efficacy benchmarks significantly reduces the risk associated with the drug's development, which is a positive indicator for future funding rounds and partnerships.
The market typically reacts favorably to promising clinical trial results, especially in the biotech sector, where the potential for high returns is substantial if the drug reaches the market. Revelation’s pathway to Phase 1b studies and the identification of a maximum tolerated dose are critical milestones that indicate the company's readiness to advance its clinical pipeline.
However, it's essential to consider the broader context. Clinical trials are expensive and the company will need substantial capital for subsequent phases. While the results are promising, investors should be aware of the inherent risks of biotech investments, including regulatory hurdles and potential competition. Overall, this development is a positive signal for the company's future prospects.
In the competitive landscape of biotechnology and drug development, Revelation Biosciences’ recent announcements position it effectively within the market. The detailed biomarker activity data showing Gemini’s immunostimulatory preconditioning effects suggest a unique approach that could differentiate it from existing therapies, particularly in niche markets like acute kidney injury (AKI) prevention.
The successful completion of Phase 1 trials not only builds credibility but also places the company on a stronger footing to negotiate partnerships and collaborations. The focus on key biomarkers like IL-1RA and NGAL, which are not only indicators of immune response but also markers of specific physiological processes, adds a layer of strategic depth. These biomarkers’ relevance to multiple indications broadens the market potential for Gemini.
Long-term, if subsequent trials continue to demonstrate safety and efficacy, Gemini could capture significant market share in the immunotherapy space. However, the company must navigate regulatory approvals and potential market competition. The ongoing development and strategic positioning will be important in determining the ultimate market impact of Gemini.
-Gemini administration induced statistically significant, dose dependent changes in key biomarkers of activity-
-Gemini was safe and well tolerated at pharmacologically active doses-
-Phase 1 results enable further development across multiple indications-
(Graphic: Business Wire)
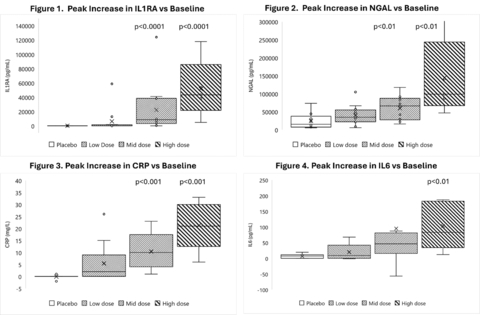
Administration of Gemini induced significant, dose dependent changes in key circulatory biomarkers of activity that reflect the expected pharmacology of Gemini-specific toll-like receptor 4 (TLR4) stimulation. Intravenous Gemini induced significant increases in interleukin-1RA (IL-1RA) (p<0.001 at mid and high dose, Figure 1), neutrophil gelatinase lipocalin (NGAL) (p<0.01 at mid and high dose, Figure 2), c-reactive protein (CRP) (p<0.001 at mid and high dose, Figure 3), and IL-6 (p<0.01 at high dose, Figure 4). Significant, dose dependent mobilization of innate immune cell populations was observed, specifically neutrophils (p<0.001 at mid-dose and high dose) and monocytes (p<0.001 at mid and high dose). Importantly, Gemini administration did not induce significant increases in serum TNF-α (p=0.51 at the highest dose) and IL-1β (p=0.89 at the highest dose). This attenuated pro-inflammatory activity and corresponding significant upregulation of beneficial cytokines is unique to Gemini and facilitates the reprogramming of the innate immune response for resolution of inflammation and promotion of the healing process.
These changes in biomarkers are consistent with the changes observed in preclinical models in which Gemini demonstrated remarkable activity (ischemia/reperfusion model of acute kidney injury and unilateral ureteral obstruction model of kidney injury) and are highly predictive of clinical efficacy, thus demonstrating the potential for Gemini in our target indications including prevention of AKI following cardiac surgery and prevention of infection following surgery.
Figures 1 through 4 illustrate the reprogramming of the innate immune response after Gemini administration in multiple biomarkers of TLR4 stimulation. IL-1RA has anti-inflammatory properties, as it binds to the IL-1 receptor, blocking IL-1α and IL-1β, major drivers of the inflammation cascade. NGAL sequesters iron and is an important defense for preventing excessive oxidative damage resulting from injury and/or ongoing inflammation. CRP plays an important role in resolving acute inflammation through increased phagocytosis, clearing cellular debris. IL-6 at low concentrations facilitates multiple activities associated with the resolution of inflammation, including stimulation of IL-1RA and IL-10. IL-10 levels trended higher with increasing dose but did not reach significance vs placebo (data not shown). While significance was not observed at the time points evaluated, IL-10 activity is inferred by the significant upregulation of IL-1RA as well as the lack of significant upregulation of TNF-α, as both activities are impacted by IL-10.
Gemini administration was generally well-tolerated. The frequency and severity of adverse events corresponded with increased dose, with the mid-level dose being established as the maximum tolerated dose in healthy volunteers. Adverse events observed included transient headache, chills, body aches/pain, and vomiting, and are consistent with preclinical findings and the expected pharmacology of the drug. Gemini administration did not result in significant changes in clinical safety markers (e.g. markers of organ function including creatinine, BUN, etc.) or hematologic parameters (aside from immune cell mobilization). There were no clinically significant findings with other safety measures including vital signs, ECG, urinalysis and physical exam. These results and the maximum tolerated dose will be used to guide dose level selection in a planned randomized, placebo-controlled Phase 1b study in patients with chronic kidney disease.
“We are pleased that the biomarker data generated in healthy volunteers is consistent with our preclinical studies. This confirms the potential utility of Gemini as a single-dose preconditioning therapy in our target indications.” said James Rolke, Chief Executive Officer of Revelation. “With this new data, we are excited and committed to moving rapidly into a Phase 1b study in patients late 2024.”
For more information on Revelation, please visit www.RevBiosciences.com.
About Gemini
Gemini is the Company’s proprietary formulation of phosphorylated hexaacyl disaccharide (PHAD®), a toll-like receptor 4 (TLR4) agonist. TLR4 stimulation with PHAD potentially preconditions the innate immune system to respond to a subsequent stress, such as ischemia (loss of blood flow) or bacterial infection. Gemini is initially being developed as a single dose preconditioning therapy for two target indications: as a pretreatment to prevent or reduce the severity of acute kidney injury due to cardiac surgery (GEMINI-AKI program) and as a pretreatment to reduce the incidence, duration, and severity of post-surgical infection (GEMINI-PSI program). In addition, Gemini has the potential to be a long-term treatment to stop or slow the progression of chronic kidney disease (GEMINI-CKD program). Revelation believes Gemini works through trained immunity, which redirects and attenuates the innate immune response to external stress (infection, trauma, etc.). Preclinical studies evaluating models of AKI or bacterial infection have demonstrated pretreatment with Gemini can reduce the severity and duration of AKI or bacterial infection, respectively. Additionally, preclinical studies evaluating a model of CKD demonstrate the potential of Gemini as a treatment to prevent kidney tissue scarring following the onset of severe inflammation.
About the Phase 1 Study Data Analysis
For all reported biomarkers except IL-1RA, the reported p-values were based on a two-tailed t-test assuming equal variances between data sets and an alpha of 0.05 for the peak change from baseline in treated subjects (by dose vs peak change in baseline in placebo subjects). For IL-1RA, a two-tailed t-test assuming equal variances between data sets and an alpha of 0.05 using a binary responder analysis of no change vs baseline and change vs. baseline was used. For each analysis there were 9 subjects in the placebo group, 12 subjects in the low dose group, 13 subjects in the mid dose group and 5 subjects in the high dose group.
About Revelation Biosciences, Inc.
Revelation Biosciences, Inc. is a clinical stage life sciences company focused on harnessing the power of trained immunity for the prevention and treatment of disease using its proprietary formulation Gemini. Revelation has multiple ongoing programs to evaluate Gemini, including as a prevention for post-surgical infection, as a prevention for acute kidney injury, and for the treatment of chronic kidney disease.
For more information on Revelation, please visit www.RevBiosciences.com.
Forward-Looking Statements
This press release contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts. These forward-looking statements are generally identified by the words "anticipate", "believe", "expect", "estimate", "plan", "outlook", and "project" and other similar expressions. We caution investors that forward-looking statements are based on management’s expectations and are only predictions or statements of current expectations and involve known and unknown risks, uncertainties and other factors that may cause actual results to be materially different from those anticipated by the forward-looking statements. Revelation cautions readers not to place undue reliance on any such forward looking statements, which speak only as of the date they were made. The following factors, among others, could cause actual results to differ materially from those described in these forward-looking statements: the ability of Revelation to meet its financial and strategic goals, due to, among other things, competition; the ability of Revelation to grow and manage growth profitability and retain its key employees; the possibility that the Revelation may be adversely affected by other economic, business, and/or competitive factors; risks relating to the successful development of Revelation’s product candidates; the ability to successfully complete planned clinical studies of its product candidates; the risk that we may not fully enroll our clinical studies or enrollment will take longer than expected; risks relating to the occurrence of adverse safety events and/or unexpected concerns that may arise from data or analysis from our clinical studies; changes in applicable laws or regulations; expected initiation of the clinical studies, the timing of clinical data; the outcome of the clinical data, including whether the results of such study is positive or whether it can be replicated; the outcome of data collected, including whether the results of such data and/or correlation can be replicated; the timing, costs, conduct and outcome of our other clinical studies; the anticipated treatment of future clinical data by the FDA, the EMA or other regulatory authorities, including whether such data will be sufficient for approval; the success of future development activities for its product candidates; potential indications for which product candidates may be developed; the ability of Revelation to maintain the listing of its securities on NASDAQ; the expected duration over which Revelation’s balances will fund its operations; and other risks and uncertainties described herein, as well as those risks and uncertainties discussed from time to time in other reports and other public filings with the SEC by Revelation.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240624937640/en/
Sandra Vedrick
Vice President, Investor Relations & Human Resources
Revelation Biosciences Inc.
Email: svedrick@revbiosciences.com
and
Chester Zygmont, III
Chief Financial Officer
Revelation Biosciences Inc.
Email: czygmont@revbiosciences.com
Source: Revelation Biosciences, Inc.







