Gemini Induces Pharmacologic Activity and Related Physiologic Changes in Multiple Preclinical Studies
- Gemini formulation shows dose-dependent increase in key biomarkers, including white blood cell migration and cytokine upregulation.
- Gemini has potential as a preventative therapy for infection and acute kidney injury.
- Phase 1 study with healthy volunteers planned to evaluate Gemini's effectiveness in humans.
- None.
Insights
Analyzing...
- Key Biomarker Activity Confirmed for Evaluation in Upcoming Phase 1 Study -
(Graphic: Business Wire)
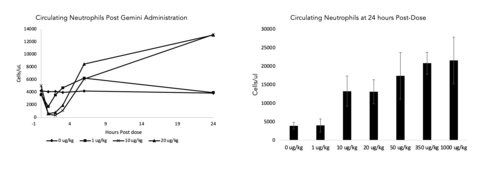
Upregulation of key biomarkers were observed in both rodent and non-rodent healthy animal preclinical studies. This included a dose dependent migration of white blood cells (neutrophils, monocytes, and lymphocytes) from the blood stream post dose with a subsequent rebound at 24 hours (data for neutrophils in non-rodent model shown in the figure below, n=3-10 per dose level examined). White blood cell mobilization and activation plays an important role in the prevention and resolution of bacterial infection.
In addition to having an effect on white blood cells, Gemini produced a dose dependent increase in multiple cytokines of interest including interleukin-10 (IL-10), neutrophil gelatinase associated lipocalin (NGAL), and interleukin-6 (IL-6). Previously on February 7, 2023 the Company announced results from a preclinical study where administration of Gemini showed the protective effect of IL-10 and NGAL upregulation on the formation of kidney scar tissue in a validated preclinical model of acute and chronic kidney injury.
IL-10 is characterized as an anti-inflammatory cytokine leading to the ultimate resolution of inflammation. NGAL (and hepcidin, data previously shown) sequester iron to prevent iron-mediated reactive oxygen tissue damage associated with inflammation and bacterial propagation by reducing available iron stores necessary for bacterial growth. Initial upregulation of IL-6 is an important first step to establish trained immunity. This phenomenon is demonstrated after the administration of two doses of Gemini which resulted in an initial increase in both inflammatory (IL-6) and protective (IL-10) cytokines following a first dose. Then an attenuated response in IL-6 following a second dose at 24 hours (e.g. a smaller increase in inflammatory cytokines and a larger increase in protective cytokines). This attenuated response in IL-6 demonstrates the premise of trained immunity and supports the application of Gemini as a preconditioning treatment to prevent excessive inflammation. The potential need for two doses to impart protection against inflammation is currently being evaluated in a preclinical model of acute kidney injury.
“We are delighted with the robust response observed following Gemini administration and look forward to demonstrating a comparable response in healthy human volunteers,” said James Rolke, Chief Executive Officer of Revelation. “A strong response in the Phase 1 study will be a very good indication of the potential of Gemini to prevent and treat diseases such as hospital acquired infection, acute kidney injury, chronic kidney disease and myocarditis in patients who currently have limited options.”
About Gemini
Gemini is a proprietary formulation for systemic administration of phosphorylated hexaacyl disaccharide (PHAD®) and is being developed as a potential therapy for prevention and treatment of hospital acquired infection (REVTx-100 program) and as a potential treatment for acute and chronic organ disease including prevention of acute kidney injury (REVTx-300 program), myocarditis, and chronic kidney disease (CKD). Revelation believes Gemini works through the process of trained immunity, comprising redirection and attenuation of the innate immune system’s response to external stress (infection, trauma, etc.). Revelation has conducted multiple preclinical studies demonstrating the therapeutic potential of Gemini in the target indications and plans to initiate clinical studies in 2023.
About Revelation Biosciences Inc.
Revelation Biosciences, Inc. is a life sciences company focused on harnessing the power of trained immunity for the prevention and treatment of disease using its proprietary formulation Gemini. Revelation has multiple ongoing programs to evaluate Gemini, including REVTx-100 as a prevention for hospital acquired infection and REVTx-300 as a prevention for acute kidney injury.
Forward-Looking Statements
This press release contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts. These forward-looking statements are generally identified by the words "anticipate", "believe", "expect", "estimate", "plan", "outlook", and "project" and other similar expressions. We caution investors that forward-looking statements are based on management’s expectations and are only predictions or statements of current expectations and involve known and unknown risks, uncertainties and other factors that may cause actual results to be materially different from those anticipated by the forward-looking statements. Revelation cautions readers not to place undue reliance on any such forward looking statements, which speak only as of the date they were made. The following factors, among others, could cause actual results to differ materially from those described in these forward-looking statements: the ability of Revelation to meet its financial and strategic goals, due to, among other things, competition; the ability of Revelation to grow and manage growth profitability and retain its key employees; the possibility that the Revelation may be adversely affected by other economic, business, and/or competitive factors; risks relating to the successful development of Revelation’s product candidates; the clinical utility of an increase in intranasal cytokine levels as a biomarker of viral infections; the ability to successfully complete planned clinical studies of its product candidates; the risk that we may not fully enroll our clinical studies or enrollment will take longer than expected; risks relating to the occurrence of adverse safety events and/or unexpected concerns that may arise from data or analysis from our clinical studies; changes in applicable laws or regulations; expected initiation of the clinical studies, the timing of clinical data; the outcome of the clinical data, including whether the results of such study is positive or whether it can be replicated; the outcome of data collected, including whether the results of such data and/or correlation can be replicated; the timing, costs, conduct and outcome of our other clinical studies; the anticipated treatment of future clinical data by the FDA, the EMA or other regulatory authorities, including whether such data will be sufficient for approval; the success of future development activities for its product candidates; potential indications for which product candidates may be developed; the potential impact that COVID-19 may have on Revelation’s suppliers, vendors, regulatory agencies, employees and the global economy as a whole; the ability of Revelation to maintain the listing of its securities on NASDAQ; investor sentiment relating to SPAC related going public transactions; the expected duration over which Revelation’s balances will fund its operations; and other risks and uncertainties described herein, as well as those risks and uncertainties discussed from time to time in other reports and other public filings with the SEC by Revelation.
View source version on businesswire.com: https://www.businesswire.com/news/home/20231012415267/en/
Sandra Vedrick
Vice President, Investor Relations & Human Resources
Revelation Biosciences, Inc.
Email: svedrick@revbiosciences.com
and
Chester Zygmont, III
Chief Financial Officer
Revelation Biosciences, Inc.
Email: czygmont@revbiosciences.com
Source: Revelation Biosciences Inc.







