AVITA Medical Announces FDA Approval of RECELL GO
AVITA Medical announced FDA approval for its advanced autologous cell harvesting device, RECELL GO. This next-gen device improves treatment for thermal burn wounds and full-thickness skin defects by harnessing a patient's own skin. Key advantages include faster healing, reduced pain, improved aesthetics, and fewer procedures. Enhanced features streamline the preparation of Spray-On Skin Cells and improve workflow efficiency. RECELL GO will launch in top U.S. burn centers in June, with broader adoption expected through the year.
- FDA approval of RECELL GO highlights regulatory success and a milestone for AVITA Medical.
- RECELL GO offers significant clinical advantages such as improved healing, reduced pain, and faster wound closures.
- Enhanced features simplify medical staff training and improve operating room efficiency.
- Planned launch in top U.S. burn centers in June indicates clear commercial strategy and potential market penetration.
- Potential for increased adoption among clinicians due to clinical and operational benefits.
- No specific financial data provided regarding potential revenue or market impact from the RECELL GO launch.
- The announcement lacks information on the cost of RECELL GO, which could affect its adoption rate.
- Possible risk of delays or complications in converting existing accounts to the new RECELL GO system.
Insights
The FDA approval of the RECELL GO™ System is a significant milestone for AVITA Medical. This approval could signal a strong market acceptance for the company's advanced regenerative medicine solutions. The ability to use significantly less donor skin while achieving improved healing and reduced pain highlights the device's potential for becoming a preferred treatment option in burn care.
Moreover, the reduced training burden on medical staff and enhanced workflow efficiency in the operating room are noteworthy. By simplifying the user interface, the device can potentially lead to broader adoption among healthcare providers, allowing them to treat more patients effectively. It's also worth noting the reduction in hospital length of stay, which can translate to lower healthcare costs and improved patient throughput.
This development is especially relevant for burn treatment centers, where any advancement that can enhance patient outcomes and streamline operations is highly valuable. The approval is likely to increase AVITA Medical's market share in the wound care sector, driving revenue growth in the short and long term.
From a financial perspective, the FDA approval of RECELL GO represents a critical catalyst for AVITA Medical's stock. Regulatory approvals often serve as key turning points for biotechnology companies, providing them with the opportunity to capitalize on new markets and drive sales growth. With RECELL GO poised to launch in top burn treatment centers in June, there is an immediate pathway to revenue generation.
Investors should watch for initial sales figures and adoption rates in these centers. Additionally, the company's strategy to convert existing accounts and provide the device to new accounts with their first order is a prudent approach to rapidly establishing market presence. This can potentially lead to a steady revenue stream and improved profit margins, given the operational efficiencies introduced by the new device.
In summary, this approval is a positive development for AVITA Medical's financial health, likely boosting investor confidence and supporting the stock's performance in the near term. Monitoring upcoming earnings reports for updates on the RECELL GO rollout will be critical for evaluating the long-term financial impact.
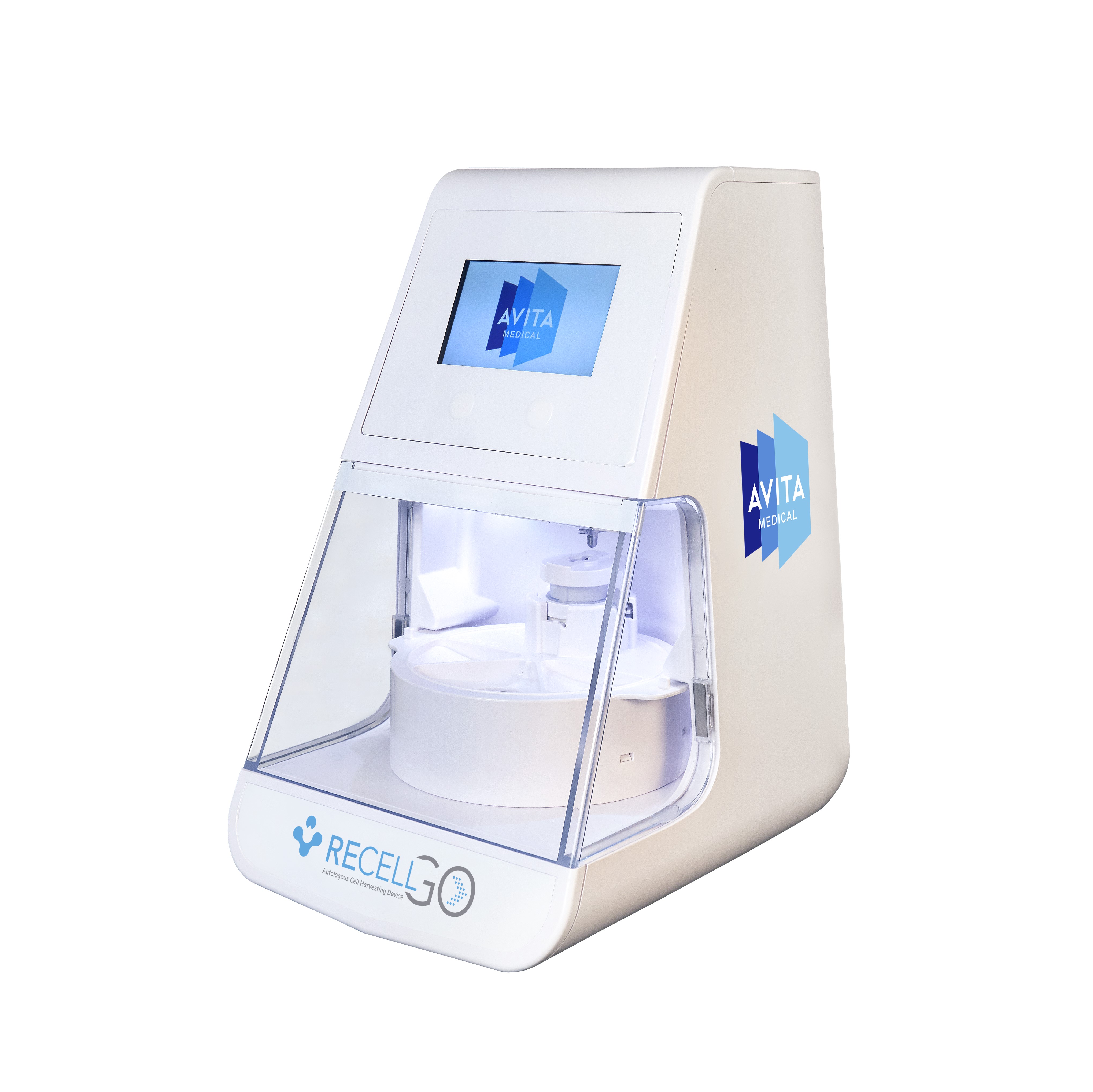
VALENCIA, Calif., May 30, 2024 (GLOBE NEWSWIRE) -- AVITA Medical, Inc. (NASDAQ: RCEL, ASX: AVH), a commercial-stage regenerative medicine company focused on first-in-class devices for wound care management and skin restoration, today announced that the U.S. Food and Drug Administration (FDA) has approved its premarket approval (PMA) supplement for the RECELL GO™ System, its next-generation autologous cell harvesting device that harnesses the regenerative properties of a patient’s own skin to treat thermal burn wounds and full-thickness skin defects.
When choosing RECELL, clinicians and patients can realize several significant advantages over traditional skin grafting:
- Improved healing is achieved using significantly less donor skin1
- Pain is reduced, closure is faster, and the aesthetic appearance at the RECELL-harvested donor site is improved2
- Fewer procedures are required for definitive closure3
- There's a reduction in the length of stay for burns covering less than
50% Total Body Surface Area (TBSA)2,3,4
RECELL GO introduces enhanced features that streamline the preparation of Spray-On Skin™ Cells. This next-generation device significantly reduces the training burden on medical staff, improves workflow efficiency in the operating room, and controls the RECELL Enzyme™ incubation time to ensure optimal cell yield and viability. These advancements simplify the user interface, enabling medical teams to provide quality care readily and consistently to their patients.
"FDA approval of RECELL GO marks a paradigm shift in the treatment of partial-thickness and full-thickness wounds,” said Jim Corbett, Chief Executive Officer of AVITA Medical. “By streamlining processes and enhancing operational efficiency with the use of RECELL GO, clinicians can now treat a greater number of patients and more broadly experience the proven benefits of RECELL technology. We believe that this transformative shift will empower more clinicians to achieve optimal outcomes for their patients, driving greater adoption, and fundamentally redefining wound care management. It's GO time for a new era in wound care."
In the United States, the Company will launch RECELL GO in its top burn treatment centers in June, and other existing accounts will be converted to RECELL GO throughout the year. New accounts will receive RECELL GO with their first order, eliminating the need for conversion.
The supplement follows the original PMA of RECELL Autologous Cell Harvesting Device and subsequent PMA supplements.
About AVITA Medical, Inc.
AVITA Medical® is a commercial-stage regenerative medicine company transforming the standard of care in wound care management and skin restoration with innovative devices. At the forefront of our platform is the RECELL® System, approved by the U.S. Food and Drug Administration for the treatment of thermal burn wounds and full-thickness skin defects, and for repigmentation of stable depigmented vitiligo lesions. RECELL harnesses the regenerative properties of a patient’s own skin to create Spray-On Skin™ Cells, delivering a transformative solution at the point-of-care. This breakthrough technology serves as the catalyst for a new treatment paradigm enabling improved clinical outcomes. AVITA Medical also holds the exclusive rights to market, sell, and distribute PermeaDerm®, a biosynthetic wound matrix, in the United States.
In international markets, the RECELL System is approved to promote skin healing in a wide range of applications including burns, full-thickness skin defects, and vitiligo. The RECELL System is TGA-registered in Australia, has received CE-mark approval in Europe and has PMDA approval in Japan.
To learn more, visit www.avitamedical.com.
Forward-Looking Statements
Statements in this announcement may contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements are subject to significant risks and uncertainties that could cause actual results to differ materially from those expressed or implied by such statements. Forward-looking statements generally may be identified by the use of words such as “anticipate,” “expect,” “intend,” “could,” “may,” “will,” “believe,” “estimate,” “look forward,” “forecast,” “goal,” “target,” “project,” “continue,” “outlook,” “guidance,” “future,” and similar words or expressions, and the use of future dates. Forward-looking statements in this announcement include but are not limited to statements concerning our product development activities, regulatory approval of our products, the potential for future growth of our business, and our ability to achieve financial goals. These statements are made as of the date of this announcement, and the Company undertakes no obligation to publicly update or revise any of these statements, except as required by law. For additional information and other important factors that may cause actual results to differ materially from forward-looking statements, please see the “Risk Factors” section of the Company’s latest Annual Report on Form 10-K and other publicly available filings for a discussion of these and other risks and uncertainties.
Authorized for release by the Chief Financial Officer of AVITA Medical, Inc.
____________________
1 Instructions for Use. RECELL® Autologous Cell Harvesting Device.
2 Holmes JH, Molnar JA, Carter JE, et al. A comparative study of the RECELL® device and autologous split-thickness meshed skin graft in the treatment of acute burn injuries. J Burn Care Res. 2018;39(5):694-702.
3 Kowal S, Kruger E, Bilir P, et al. Cost effectiveness of the use of autologous cell harvesting device compared to standard of care for treatment of severe burns in the United States. Adv Ther. Published online May 7, 2019. doi:10.1007/s12325-019-00961-2.
4 Holmes JH, Molnar JA, Carter JE, et al. A comparative study of the RECELL® device and autologous split-thickness meshed skin graft in the treatment of acute burn injuries. J Burn Care Res. 2018;39(5):694-702.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/5e843520-955d-4f45-a7f8-53b0a6f3a3f6








