Pulse Biosciences Announces First CellFX Procedures Performed in Europe as Part of Global Controlled Launch
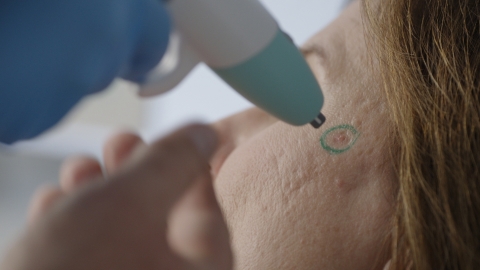
Pulse Biosciences (Nasdaq: PLSE) has successfully completed the first CellFX procedures in the European Union, marking a critical milestone in its controlled launch program. The CellFX System, which utilizes Nano-Pulse Stimulation™ technology, is designed to non-thermally treat common benign lesions, enhancing patient satisfaction. A survey highlighted that patients prioritize treatment for skin lesions, with an average of 200 cases per month per aesthetic dermatologist. The company aims to establish best practices with leading specialists across Europe, while concurrently launching in the U.S.
- Successful completion of first CellFX procedures in the EU.
- Expansion of controlled launch program targeting aesthetic dermatologists and plastic surgeons.
- High market demand for treatments addressing benign lesions.
- Positive feedback from practitioners about the CellFX System's innovative approach.
- None.
Pulse Biosciences, Inc. (Nasdaq: PLSE), a novel bioelectric medicine company progressing Nano-Pulse Stimulation™ (NPS™) technology, today announced that the first CellFX® procedures in the European Union were successfully completed. The initial commercial use of the non-thermal, cellular-focused CellFX System to clear common benign lesions, now expands the Company’s controlled launch program starting with the top aesthetic dermatologists and plastic surgeons across Europe. This strategic rollout in Europe will run in parallel with the Company’s U.S. controlled launch aimed at building a strong foundation of clinical and commercial advocacy as the Company grows a promising global business.
(Photo: Business Wire)
As the first-of-its-kind multi-application platform powered by NPS technology delivering nano-second pulses of electrical energy to non-thermally clear cells while protecting adjacent non-cellular healthy tissue, the CellFX System ushers in a new chapter in dermatology procedures addressing everyday skin lesions.
“I am delighted to be the first aesthetic skin specialist to perform the CellFX procedure in Europe. We see a huge number of patients who are burdened by troublesome skin lesions, such as sebaceous hyperplasia, seborrheic keratoses, and non-genital warts, that can affect them at home, work and socially,” said Dr. Afschin Fatemi, medical director of The S-thetic Group, a network of aesthetic clinics across Germany. “With its non-thermal, cellular mechanism, the CellFX System provides a new, consistent solution to remove a variety of bumps and growths on the face and body that have been historically difficult to treat. I am excited to offer this innovative procedure in our clinics and look forward to the enhanced results and improved patient satisfaction we can expect to achieve.”
The prevalence of SH, SK and common, non-genital warts among patients visiting aesthetic dermatologists today is widespread. Based on a 2020 survey among aesthetic physicians from Germany, Spain and France, an average of 200 patients per month who visit aesthetic dermatology practices present with each lesion type (SH, SK, non-genital warts). Further, patients place greater value on a procedure to treat skin lesions over other popular aesthetic procedures they currently receive and are willing to pay cash to treat multiple lesions in a single visit.1
"We are at the inception of a distinctive new era as we embark on the commercial use of the CellFX System for specific lesion types throughout the European Union. In partnership with leading aesthetic skin specialists across Europe, our ambition is to create a blueprint of best practices that ensures success for the next wave of early adopters,” said Ed Ebbers, Executive Vice President and General Manager, Dermatology, of Pulse Biosciences. “We are honored to work with Dr. Fatemi and we look forward to a promising future in the European market.”
About Pulse Biosciences®
Pulse Biosciences is a novel bioelectric medicine company committed to health innovation that has the potential to improve the quality of life for patients. The CellFX® System is the first commercial product to harness the distinctive advantages of the Company’s proprietary Nano-Pulse StimulationTM (NPSTM) technology, such as the ability to non-thermally clear cells while sparing non-cellular tissue, to treat a variety of applications for which an optimal solution remains unfulfilled. Nano-Pulse Stimulation technology delivers nano-second pulses of electrical energy. The initial commercial use of the CellFX System is to address a range of dermatologic conditions that share high demand among patients and practitioners for improved dermatologic outcomes. Designed as a multi-application platform, the CellFX System offers customer value with a utilization-based revenue model. To learn more, please visit pulsebiosciences.com.
To stay informed about the CellFX System, please visit CellFX.com and sign up for updates.
Pulse Biosciences, CellFX, Nano-Pulse Stimulation, NPS and the stylized logos are among the trademarks and/or registered trademarks of Pulse Biosciences, Inc. in the United States and other countries.
Forward-Looking Statements
All statements in this press release that are not historical are forward-looking statements, including, among other things, statements relating to Pulse Biosciences’ expectations regarding regulatory clearance and the timing of FDA, Health Canada and other regulatory filings or approvals, and the ability of the Company to successfully complete a 510(k) submission for the CellFX System or for any specific indications, the ability of the Company to prepare and provide data to FDA, Canadian and other regulatory bodies, NPS technology including the effectiveness of such technology and the effectiveness of related clinical studies in predicting outcomes resulting from the use of NPS technology, the CellFX System including the benefits of the CellFX System and commercialization of the CellFX System, current and planned future clinical studies and the ability of the Company to execute such studies and results of any such studies, other matters related to its pipeline of product candidates, the Company’s market opportunity and commercialization plans, including the timing and results of the controlled launch in Europe, the market for the treatment of certain lesions, the experience of using the CellFX System, future financial performance, and other future events. These statements are not historical facts but rather are based on Pulse Biosciences’ current expectations, estimates, and projections regarding Pulse Biosciences’ business, operations and other similar or related factors. Words such as “may,” “will,” “could,” “would,” “should,” “anticipate,” “predict,” “potential,” “continue,” “expects,” “intends,” “plans,” “projects,” “believes,” “estimates,” and other similar or related expressions are used to identify these forward-looking statements, although not all forward-looking statements contain these words. You should not place undue reliance on forward-looking statements because they involve known and unknown risks, uncertainties, and assumptions that are difficult or impossible to predict and, in some cases, beyond Pulse Biosciences’ control. Actual results may differ materially from those in the forward-looking statements as a result of a number of factors, including those described in Pulse Biosciences’ filings with the Securities and Exchange Commission. Pulse Biosciences undertakes no obligation to revise or update information in this release to reflect events or circumstances in the future, even if new information becomes available.
1 2020 Physician (n=46) and Patient (n=190) surveys conducted in the EU by third-party market research firm on behalf of Pulse Biosciences, Inc.
View source version on businesswire.com: https://www.businesswire.com/news/home/20210219005136/en/
FAQ
What is the significance of Pulse Biosciences' recent announcement regarding the CellFX System in the EU?
How does the CellFX System utilize Nano-Pulse Stimulation technology?
What types of skin lesions can the CellFX System treat?







