Precigen Announces Positive Phase 1 Dose Escalation Data for Autologous PRGN-3006 UltraCAR-T® Manufactured Overnight for Next Day Infusion in Relapsed or Refractory Acute Myeloid Leukemia Patients
Precigen announced positive Phase 1 data for PRGN-3006 UltraCAR-T at the ASH Annual Meeting. The treatment showed a 27% objective response rate (ORR) in heavily pre-treated relapsed/refractory acute myeloid leukemia (AML) patients following lymphodepletion. Notably, 60% of patients experienced a decrease in bone marrow blasts. The therapy was well-tolerated with no dose-limiting toxicities reported, highlighting its safety profile. PRGN-3006 has received both Orphan Drug and Fast Track Designations from the FDA, underscoring its potential in addressing critical cancer treatment gaps.
- 27% objective response rate (ORR) in heavily pre-treated r/r AML patients after lymphodepletion.
- 60% of patients in the lymphodepletion cohort showed a reduction in bone marrow blasts.
- Well-tolerated treatment with no dose-limiting toxicities reported.
- PRGN-3006 received Orphan Drug and Fast Track Designations from the FDA.
- None.
Insights
Analyzing...
– Single infusion of UltraCAR-T cells with or without lymphodepletion demonstrated robust expansion and persistence in blood and bone marrow –
– PRGN-3006 infusion with lymphodepletion resulted in a decrease in bone marrow blasts in
– Single infusion of autologous PRGN-3006 cells resulted in
– PRGN-3006 was well-tolerated with no dose-limiting toxicities (DLTs) reported to date –
GERMANTOWN, Md., Dec. 12, 2022 /PRNewswire/ -- Precigen, Inc., a biopharmaceutical company specializing in the development of innovative gene and cell therapies to improve the lives of patients, today presented positive Phase 1 dose escalation data from the ongoing Phase 1/1b clinical study of PRGN-3006 UltraCAR-T® in patients with r/r AML and higher risk myelodysplastic syndromes (MDS) (clinical trial identifier: NCT03927261) at the 64th American Society of Hematology (ASH) Annual Meeting and Exposition (Abstract# 4633). The presentation was delivered by David A. Sallman, MD, Assistant Member in the Department of Malignant Hematology at the H. Lee Moffitt Cancer Center & Research Institute (Moffitt) and a lead investigator for the PRGN-3006 clinical trial.
PRGN-3006 UltraCAR-T is a multigenic autologous chimeric antigen receptor (CAR)-T simultaneously expressing a CAR specifically targeting CD33; membrane bound IL-15 (mbIL15) for enhanced in vivo expansion and persistence; and a kill switch to conditionally eliminate CAR-T cells for an improved safety profile. CD33 is over-expressed on AML blasts with lesser expression on normal hematopoietic stem cells. PRGN-3006 UltraCAR-T drug product is manufactured via an overnight process at medical centers using the Company's proprietary non-viral and UltraPorator® systems and released for infusion in patients the next day. The decentralized, overnight UltraCAR-T manufacturing process, which does not use viral vectors or ex vivo activation and expansion of T cells, has the potential to address major limitations of current T cell therapies. PRGN-3006 UltraCAR-T has been granted Orphan Drug Designation and Fast Track Designation in patients with AML by the US Food and Drug Administration (US FDA).
The Phase 1/1b clinical study is designed to enroll in two phases, an initial dose escalation phase followed by a dose expansion phase, to evaluate safety and determine the recommended Phase 2 dose of PRGN-3006 delivered via intravenous (IV) infusion without lymphodepletion (Cohort 1) or with lymphodepletion (Cohort 2). The study is also evaluating in vivo persistence and anti-tumor activity of PRGN-3006.
Today's ASH presentation includes the complete data set for the Phase 1 dose escalation phase of the study. The study enrolled a total of 26 patients (N=10 non-lymphodepletion; N=16 with lymphodepletion) and included 21 patients with r/r AML, 2 patient with chronic myelomonocytic leukemia (CMML), and 3 patients with MDS. The median age was 60.5 years (range: 32-77). Patients were heavily pre-treated with a median of 3.5 prior regimens (range: 1-9) and
"The dose escalation data for PRGN-3006 showed robust dose-dependent expansion and persistence of PRGN-3006 in peripheral blood and bone marrow following a single infusion with no DLTs reported to date leading to an ORR of
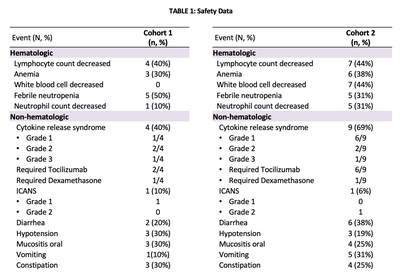
Safety Data
Cohort 1: Non-lymphodepletion
PRGN-3006 was well-tolerated with no dose-limiting toxicities (DLTs) reported to date in the cohort without lymphodepletion (TABLE 1). Overall, there was a low incidence of adverse events following PRGN-3006 infusion without lymphodepletion and the most common adverse events were decreased lymphocyte count, anemia, febrile neutropenia, cytokine release syndrome (CRS), hypotension and oral mucositis. The majority of treatment emergent adverse events (TEAEs) were either Grade 1 or 2 with only one transient Grade 3 CRS reported (Dose Level 1), which resolved in less than 24 hours with tocilizumab and dexamethasone. Other cases of CRS were Grade 1 or 2 and required either no intervention or resolved following standard CRS management. One Grade 1 immune effector cell-associated neurotoxicity syndrome (ICANS) was reported. No patients experienced a significant increase in serum IL-15, demonstrating that mbIL15 remains tethered to the UltraCAR-T cells as designed and is not released.
Cohort 2: Lymphodepletion
In the lymphodepletion cohort, PRGN-3006 was also well-tolerated with no DLTs reported to date (TABLE 1). Overall, there was a low incidence of adverse events following PRGN-3006 infusion with lymphodepletion and the most common adverse events were decreases in lymphocytes, white blood cells and neutrophil, anemia, febrile neutropenia and CRS. The majority of TEAEs were either Grade 1 or 2. Only one Grade 3 CRS was reported (Dose Level 3), which was subsequently downgraded to Grade 1 by the investigator. Other cases of CRS were Grade 1 or 2 and required either no intervention or resolved following standard CRS management. One Grade 2 ICANS was reported. No patients experienced a significant increase in serum IL-15, demonstrating that mbIL15 remains tethered to the UltraCAR-T cells as designed and is not released.
Clinical Activity
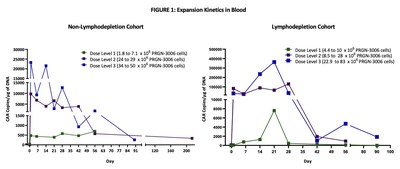
Expansion Kinetics
Excellent dose-dependent expansion and persistence of PRGN-3006 in peripheral blood and bone marrow was observed following a single infusion in both the non-lymphodepletion and lymphodepletion cohorts highlighting the ability of UltraCAR-T cells to engraft and survive even in the absence of lymphodepletion. Higher peak expansion (> 10 fold) in peripheral blood was observed in the lymphodepletion cohort compared to non-lymphodepletion cohort at the same dose level (FIGURE 1).
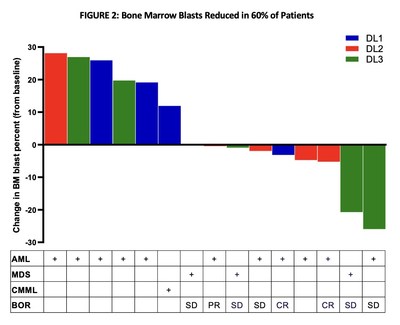
Change in Bone Marrow Blasts
Of the 15 evaluable patients in the lymphodepletion cohort,
Objective Responses
Cohort 1: Non-lymphodepletion
In the non-lymphodepletion cohort, 3 out of 10 patients had Stable Disease (SD), per European LeukemiaNet (ELN) criteria, persisting for more than 3 months with one patient experiencing durable SD for more than 7 months with concomitant reduction in peripheral blast levels.
Cohort 2: Lymphodepletion
An objective response rate (ORR) of
TABLE 2: Summary of Objective Responses for the Lymphodepletion Cohort | |||
AML | MDS | CMML | |
Disease Control Rate (at D28) | 5/11 ( | 3/3 ( | 0/1 |
Objective Response Rate (ORR) | 3/11 ( | 0/3 | 0/1 |
TABLE 3: Summary Data for Objective Responders | |||||
AML Subtype | Age | Sex | Prior Regimens* | Safety** | Objective Response*** |
Persistent AML | 60 | F | 2 prior: CLAG and HiDAC | No incidence of CRS, neurotoxicity or DLT | CRh at Day 84 Allo-HSCT at Month 3; Surviving 18 months post-transplant |
Extramedullary AML | 53 | M | 7 prior: intensive chemo, vidasia, venetoclax, FLAG, anti-IDH1, allo-HSCT | No incidence of CRS, neurotoxicity or DLT | PR at Day 28 PR at 60 |
AML | 61 | F | 4 prior: vyxeos, HMA+venetoclax, allo-HSCT | CRS Grade 1, with SAE skin rash, (possible GVHD) | CRi at Day 28 CRh at Day 60 |
*CLAG=cladribine, cytarabine, and granulocyte-stimulating factor; HiDAC=high-dose cytarabine; FLAG=fludarabine, cytarabine and filgrastim; anti-IDH1=isocitrate dehydrogenases 1 inhibitor; HMA=hypomethylating agents (HMA); allo-HSCT= allogeneic hematopoietic stem cell transplant |
**SAE=small ubiquitin-like modifier activating enzyme; GVHD=graft versus host disease |
***(CRi) Complete Response with incomplete hematologic recovery (per ELN criteria; (CRh) Complete response with hematologic recovery per ELN criteria; PR: partial response RECIST v1.1 |
Analysis of peripheral blood samples post PRGN-3006 infusion showed gene expression changes consistent with improvement in the immune compartment function for anti-tumor effect in responders. There was an increase in cytotoxicity, costimulatory signaling, and lymphoid compartment and decreased apoptosis pathway scores in the lymphodepletion cohortA on Days 14 and 28 post PRGN-3006 treatment compared to baseline. Furthermore, preliminary analysis shows a potential correlation between a biomarker and objective responses at different dose levels in AML patients, which will be further investigated in the ongoing Phase 1b expansion trial.
PRGN-3006 is currently being evaluated following lymphodepletion in the multicenter Phase 1b dose expansion phase of the study. In the dose expansion phase, patients can receive repeat dosing of PRGN-3006. There is no requirement for additional lymphodepletion in repeat dose patients due to the demonstrated ability of PRGN-3006 to expand in the absence of lymphodepletion.
"We are pleased with the performance of PRGN-3006 UltraCAR-T in demonstrating meaningful clinical responses for heavily pre-treated r/r AML patients who have limited therapeutic options. These data further validate our innovative approach of overnight, decentralized manufacturing of autologous CAR-T cells and demonstrate the capability of the UltraCAR-T platform to directly expand in vivo and persist in the body leading to complete and partial responses in cancer patients with highly advanced disease," said Helen Sabzevari, PhD, President and CEO of Precigen. "We believe the UltraCAR-T platform is distinctly differentiated from other cell therapy technologies with the potential to bring cutting-edge treatments to all cancer patients rapidly and economically."
Precigen: Advancing Medicine with Precision™
Precigen (Nasdaq: PGEN) is a dedicated discovery and clinical stage biopharmaceutical company advancing the next generation of gene and cell therapies using precision technology to target the most urgent and intractable diseases in our core therapeutic areas of immuno-oncology, autoimmune disorders, and infectious diseases. Our technologies enable us to find innovative solutions for affordable biotherapeutics in a controlled manner. Precigen operates as an innovation engine progressing a preclinical and clinical pipeline of well-differentiated therapies toward clinical proof-of-concept and commercialization. For more information about Precigen, visit www.precigen.com or follow us on Twitter @Precigen, LinkedIn or YouTube.
About Acute Myeloid Leukemia (AML)
AML is a cancer that starts in the bone marrow, but most often moves into the blood.1 Though considered rare, AML is among the most common types of leukemia in adults.2 In 2019, it was estimated that 21,450 new cases of AML would be diagnosed in the US.2 AML is uncommon before the age of 45 and the average age of diagnosis is about 68.2 The prognosis for patients with AML is poor with an average 5‐year survival rate of approximately 25 percent overall, and less than a 5 percent 5‐year survival rate for patients older than 65.3 Amongst elderly AML patients (≥ 65 years of age), median survival is short, ranging from 3.5 months for patients 65 to 74 years of age to 1.4 months for patients ≥ 85 years of age.3
About Myelodysplastic Syndrome (MDS)
MDS are diseases of the bone marrow generally found in adults in their 70s.4 Incidence in the US is not known for sure, but estimates range from 10,000 each year and higher.4 Using International Prognostic Scoring System (IPSS-R), median survival for MDS patients can vary from less than one year for the "very high" IPSS-R risk group to more than eight years for the "very low" IPSS-R group.4
UltraCAR-T®
UltraCAR-T is a multigenic autologous CAR-T platform that utilizes Precigen's advanced non-viral Sleeping Beauty system to simultaneously express an antigen-specific CAR to specifically target tumor cells, mbIL15 for enhanced in vivo expansion and persistence, and a kill switch to conditionally eliminate CAR-T cells for a potentially improved safety profile. Precigen has advanced the UltraCAR-T platform to address the inhibitory tumor microenvironment by incorporating a novel mechanism for intrinsic checkpoint blockade without the need for complex and expensive gene editing techniques. UltraCAR-T investigational therapies are manufactured via Precigen's overnight manufacturing process using the proprietary UltraPorator electroporation system at the medical center and administered to patients only one day following gene transfer. The overnight UltraCAR-T manufacturing process does not use viral vectors and does not require ex vivo activation and expansion of T cells, potentially addressing major limitations of current T cell therapies.
UltraPorator®
The UltraPorator system is an exclusive device and proprietary software solution for the scale-up of rapid and cost-effective manufacturing of UltraCAR-T therapies and potentially represents a major advancement over current electroporation devices by significantly reducing the processing time and contamination risk. The UltraPorator device is a high-throughput, semi-closed electroporation system for modifying T cells using Precigen's proprietary non-viral gene transfer technology. UltraPorator is being utilized for clinical manufacturing of Precigen's investigational UltraCAR-T therapies in compliance with current good manufacturing practices.
Trademarks
Precigen, UltraCAR-T, UltraPorator and Advancing Medicine with Precision are trademarks of Precigen and/or its affiliates. Other names may be trademarks of their respective owners.
Cautionary Statement Regarding Forward-Looking Statements
Some of the statements made in this press release are forward-looking statements. These forward-looking statements are based upon the Company's current expectations and projections about future events and generally relate to plans, objectives, and expectations for the development of the Company's business, including the timing and progress of preclinical studies, clinical trials, discovery programs and related milestones, the promise of the Company's portfolio of therapies, and in particular its CAR-T and AdenoVerse therapies. Although management believes that the plans and objectives reflected in or suggested by these forward-looking statements are reasonable, all forward-looking statements involve risks and uncertainties, including the possibility that the timeline for the Company's clinical trials might be impacted by the COVID-19 pandemic, and actual future results may be materially different from the plans, objectives and expectations expressed in this press release. The Company has no obligation to provide any updates to these forward-looking statements even if its expectations change. All forward-looking statements are expressly qualified in their entirety by this cautionary statement. For further information on potential risks and uncertainties, and other important factors, any of which could cause the Company's actual results to differ from those contained in the forward-looking statements, see the section entitled "Risk Factors" in the Company's most recent Annual Report on Form 10-K and subsequent reports filed with the Securities and Exchange Commission.
References
1 American Cancer Society. What is Acute Myeloid Leukemia (AML)?
2 American Cancer Society. Key Statistics for Acute Myeloid Leukemia (AML)
3 Thein, M., et al., Outcome of older patients with acute myeloid leukemia: an analysis of SEER data over 3 decades. Cancer, 2013. 119(15): p.2720-7
4 American Cancer Society. Key Statistics for Myelodysplastic Syndromes
Investor Contact:
Steven Harasym
Vice President, Investor Relations
Tel: +1 (301) 556-9850
investors@precigen.com
Media Contacts:
Donelle M. Gregory
press@precigen.com
Glenn Silver
Lazar-FINN Partners
glenn.silver@finnpartners.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/precigen-announces-positive-phase-1-dose-escalation-data-for-autologous-prgn-3006-ultracar-t-manufactured-overnight-for-next-day-infusion-in-relapsed-or-refractory-acute-myeloid-leukemia-patients-301700832.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/precigen-announces-positive-phase-1-dose-escalation-data-for-autologous-prgn-3006-ultracar-t-manufactured-overnight-for-next-day-infusion-in-relapsed-or-refractory-acute-myeloid-leukemia-patients-301700832.html
SOURCE Precigen, Inc.