SARS-CoV-2 Spike Protein Licensed by Oragenics from the NIH Demonstrates Protective Immunity in Mice
Oragenics, Inc. (NYSE American: OGEN) (“Oragenics” or the “Company”) announces that the stabilized pre-fusion spike protein CoV-2 S-2P created by the National Institutes of Health (“NIH”) and licensed by the Company demonstrates protective immunity in immunized mice challenged with mouse-adapted SARS-CoV-2 virus.
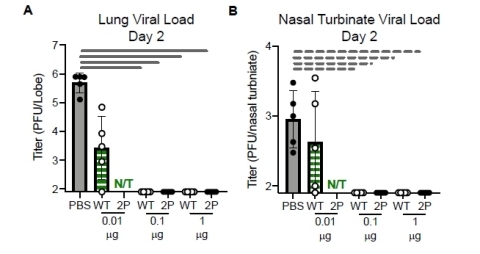
Gray dashed line = p<0.05 Gray solid line = p<0.01 (Graphic: Business Wire)
The NIH data shows that the NIH – created COVID-19 spike protein when dosed at either 0.1 or 1.0 mcg and combined with an adjuvant completely inhibited virus growth in the nasal cavities and lungs of mice when the animals were infected four weeks after their second immunization, compared to the unvaccinated control animals. The adjuvant uses was TLR-4-agonist Sigma Adjuvant System - a TLR-4 agonist that induces T cell activation in mice. Adjuvants are added to vaccines to boost immune responses.
See Dinnon et al for details on the mouse-adapted challenge model - Dinnon et al, Nature 586, 560–566 (2020) located at - https://doi.org/10.1038/s41586-020-2708-8.
The Company previously disclosed that the NIH-produced spike protein adjuvanted with the TLR-4-agonist Sigma Adjuvant System generated neutralizing antibody titers, measured using a pseudovirus neutralization assay and a plaque-reduction neutralization titer assay. In addition, this immunization produced a balanced Th1/Th2 (T helper cells) response. In a well-functioning immune system both groups of T helper cells work together to keep the system balanced.
“We are encouraged that our licensed SARS-CoV-2 spike protein has been shown to hold promise in the creation of a COVID-19 vaccine, and believe these additional animal data supports our continued development of Terra CoV-2, our lead COVID-19 vaccine candidate,” said Alan Joslyn, Ph.D., President and Chief Executive Officer of Oragenics. Dr. Joslyn continued, “Our successful pre-IND meeting with the FDA has allowed us a clear path towards an Investigational New Drug application. We continue to aggressively move forward with our development strategy for this vaccine candidate, as we believe the global need for vaccines against SARS-CoV-2 is estimated to be between 12 billion to 14 billion doses and the vaccines currently being administered are only a first step towards addressing the COVID-19 pandemic.”
About Oragenics, Inc.
Oragenics, Inc. is focused on the creation of the Terra CoV-2 development of effective treatments for novel antibiotics against infectious disease. The Company is dedicated to the development and commercialization of a vaccine candidate providing specific immunity from novel coronavirus. The Terra CoV-2 immunization leverages coronavirus spike protein research conducted by the National Institute of Health. In addition, Oragenics has an exclusive worldwide channel collaboration with ILH Holdings, Inc. (n/k/a Eleszto Genetika, Inc.), relating to the development of novel lantibiotics.
Forward-Looking Statements
This communication contains “forward-looking statements” within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on management’s beliefs and assumptions and information currently available. The words "believe," "expect," "anticipate," "intend," "estimate," "project" and similar expressions that do not relate solely to historical matters identify forward-looking statements. Investors should be cautious in relying on forward-looking statements because they are subject to a variety of risks, uncertainties, and other factors that could cause actual results to differ materially from those expressed in any such forward-looking statements. These factors include, but are not limited to, the following: the Company’s ability to advance the development of Terra CoV-2 under the timelines and in accord with the milestones it projects; the Company’s ability to obtain funding, non-dilutive or otherwise, for the development of Noachis Terra’s Terra CoV-2 vaccine, whether through its own cash on hand, or another alternative source; the regulatory application process, research and development stages, and future clinical data and analysis relating to Terra CoV-2, including any meetings, decisions by regulatory authorities, such as the FDA and investigational review boards, whether favorable or unfavorable; the potential application of Terra CoV-2 to other coronaviruses; the Company’s ability to obtain, maintain and enforce necessary patent and other intellectual property protection; the nature of competition and development relating to COVID-19 immunization and therapeutic treatments and demand for vaccines; the Company’s expectations as to storage and distribution, potential market and impact of other vaccines being administered; other potential adverse impacts due to the global COVID-19 pandemic, such as delays in regulatory review, interruptions to manufacturers and supply chains, adverse impacts on healthcare systems and disruption of the global economy; and general economic and market conditions risks, as well as other uncertainties described in our filings with the U.S. Securities and Exchange Commission. All information set forth in this press release is as of the date hereof. You should consider these factors in evaluating the forward-looking statements included in this press release and not place undue reliance on such statements. We do not assume any obligation to publicly provide revisions or updates to any forward-looking statements, whether as a result of new information, future developments or otherwise, should circumstances change, except as otherwise required by law.
View source version on businesswire.com: https://www.businesswire.com/news/home/20210202005441/en/







