Orthofix Announces US and European Full Market Launch of FITBONE Limb-Lengthening System
Orthofix Medical Inc. (NASDAQ:OFIX) announced the full market launch of its FITBONE® intramedullary lengthening system in the U.S. and Europe for femur and tibia deformity corrections. The system, which is fully implantable, has been used in over 3,500 cases across 15 countries. It gained U.S. FDA 510(k) clearance and CE Mark approval in Europe. This expansion aims to improve treatment options for surgeons and patients. Orthofix offers a comprehensive portfolio of fixation solutions, enhancing its market presence in orthopedic care.
- Successful launch of FITBONE® system in U.S. and Europe, expanding market access.
- Over 3,500 cases performed, indicating strong acceptance and potential market demand.
- FDA 510(k) clearance and CE mark approval enhance product credibility.
- Potential slow adoption by surgeons may hinder market penetration.
- Risk that future studies may not support expected patient outcomes.
- Competition could limit market share if superior products emerge.
Insights
Analyzing...
Orthofix Medical Inc. (NASDAQ:OFIX), a global medical device company with a spine and extremities focus, today announced the U.S. and European full market launch of the FITBONE® intramedullary lengthening system for limb lengthening and deformity correction of the femur and tibia bones.
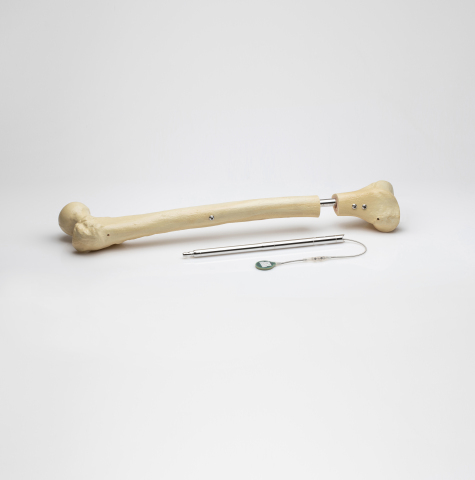
Image of the FITBONE® intramedullary lengthening system for limb lengthening and deformity correction of the femur and tibia bones. (Image provided by Orthofix. )
“Orthofix is the only orthopedic company that offers a comprehensive portfolio of both internal and external fixation solutions for limb reconstruction and deformity correction,” said Orthofix President of Global Extremities Paul Gonsalves. “We are pleased to now be able to broaden access to the FITBONE system by expanding availability in the U.S. and Europe, giving surgeons a choice in treating patients in need of deformity correction and limb lengthening.”
The FITBONE intramedullary lengthening nail is a fully implantable system for correcting leg length and deformity discrepancies. Implanted through a minimally invasive procedure, the system consists of the implanted intramedullary nail, a subcutaneously implanted receiver and an external control set that enables the patient to manage the distraction phase at home.
The system is designed to provide accurate and controlled limb lengthening, with more than 3,500 cases performed in 15 countries since its development. With appropriate preoperative planning, it allows achievement of axial and torsional bone alignment intraoperatively, as part of the limb-lengthening procedure.
The FITBONE intramedullary lengthening system is available in the U.S. under a U.S. Food and Drug Administration 510(k) clearance and in European Countries under a CE Mark approval.
The system is a part of the Orthofix Extremities portfolio of solutions that include the TL-HEX TrueLok Hexapod System™ computer-assisted ring fixation system for external limb lengthening, JPS JuniOrtho Plating system with the OrthoNext™ digital surgery planning software and the eight-Plate Guided Growth System™ for correcting angular growth deformities in pediatric patients. To learn more about Orthofix’s dedication to helping surgeons and limb deformity correction patients, please visit www.Orthofix.com.
About Orthofix
Orthofix Medical Inc. is a global medical device and biologics company with a spine and extremities focus. The Company’s mission is to deliver innovative, quality-driven solutions as we partner with health care professionals to improve patients’ lives. Headquartered in Lewisville, Texas, Orthofix’s spine and orthopedic extremities products are distributed in more than 70 countries via the Company's sales representatives and distributors. For more information, please visit www.Orthofix.com.
Forward-Looking Statements
This communication contains forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended, relating to our business and financial outlook, which are based on our current beliefs, assumptions, expectations, estimates, forecasts and projections. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “projects,” “intends,” “predicts,” “potential,” or “continue” or other comparable terminology. These forward-looking statements are not guarantees of our future performance and involve risks, uncertainties, estimates and assumptions that are difficult to predict, including the risks described in Part II Item 1A under the heading Risk Factors of our Quarterly Report on Form 10-Q for the quarter ended September 30, 2020, and Part I, Item 1A under the heading Risk Factors in our Annual Report on Form 10-K for the year ended December 31, 2019 (the “2019 Form 10-K”). In addition to the risks described there, factors that could cause or contribute to such differences may include, but are not limited to: the risk that surgeons may be slow to adopt the FITBONE intramedullary lengthening system ; the risk that future patient studies or clinical experience and data may indicate that treatment with the FITBONE system does not improve patient outcomes as much as previously believed, or otherwise call into question the benefits of its use to patients, hospitals and surgeons; the risk that the product may not perform as intended and may therefore not achieve commercial success; the risk that competitors may develop superior products or may have a greater market position enabling more successful commercialization; the risk that insurance payers may decline to reimburse healthcare providers for the use of our products.
This list of risks, uncertainties and other factors is not complete. We discuss some of these matters more fully, as well as certain risk factors that could affect our business, financial condition, results of operations, and prospects, in reports we file from time-to-time with the Securities and Exchange Commission, which are available to read at www.sec.gov. Any or all forward-looking statements that we make may turn out to be wrong (due to inaccurate assumptions that we make or otherwise), and our actual outcomes and results may differ materially from those expressed in these forward-looking statements. You should not place undue reliance on any of these forward-looking statements. Further, any forward-looking statement speaks only as of the date hereof, unless it is specifically otherwise stated to be made as of a different date. We undertake no obligation to update, and expressly disclaim any duty to update, our forward-looking statements, whether as a result of circumstances or events that arise after the date hereof, new information, or otherwise.
View source version on businesswire.com: https://www.businesswire.com/news/home/20210211005237/en/







