Orthofix Announces First Patient Procedures and Full Commercial Launch of the Galaxy Fixation Gemini System and Expanded Sterile Kit Offerings for Orthopedic Trauma Procedures
- The Galaxy Fixation Gemini system provides a quick off-the-shelf solution for treating fractures resulting from trauma in limbs, improving response time in trauma settings.
- The sterile kits eliminate spatial constraints and reduce costs for healthcare facilities by eliminating the need for sterilization of trays.
- The ankle kit in particular, with specific clamps available in a sterile kit configuration, offers more efficient solutions in lower extremity trauma situations where time is crucial.
- None.
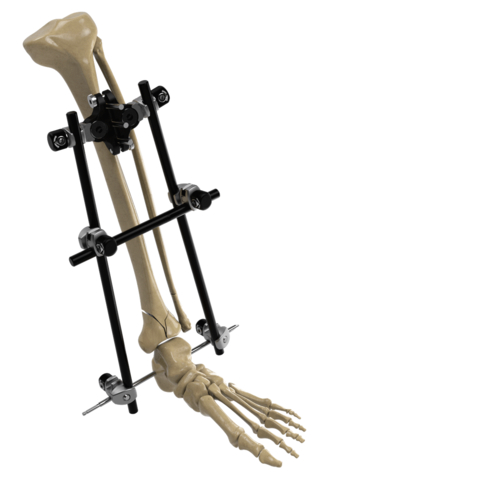
Image of the Galaxy Fixation Gemini System for treating fractures that result from trauma in the lower and upper limbs. (Photo: Business Wire)
“In trauma settings, it is critical that we respond quickly to manage the damage to the impacted limb,” said Dr. Evengy Dyskin, Clinical Assistant Professor of Orthopaedics, University of
Sterile kits eliminate the need for sterilization of trays which takes an average of 139 minutes1 to process and adds cost to healthcare facilities. Sterilization of trays also results in increased operating room (OR) delays and costs due to packaging, restocking and tray contamination. Average cost to a facility for OR delays related to tray contamination is
In particular, the Galaxy Fixation Gemini ankle kit is the only pin-to-bar system with specific clamps available in a sterile kit configuration giving surgeons more efficient solutions in lower extremity trauma situations where time is an important factor. The ankle kit, developed specifically to meet the needs of
“We are excited to expand our external fixation offerings in the
Orthofix further expanded its sterile kit offerings with the global launch of the CalcFix Plus Calcaneal Minifixator™ System earlier this year. The CalcFix Plus represents the latest version of the original CalcFix Fixator device designed to treat calcaneal fractures using a more minimally invasive external fixation approach compared with internal fixation and includes updates to the design that reduce the number of operative steps to apply.
The Galaxy Fixation Gemini and the CalcFix Plus Systems add to Orthofix’s extensive portfolio of over 50 sterile kit procedural solutions available globally and demonstrate Orthofix’s continued investment in providing off-the-shelf solutions to meet the needs in the acute trauma, military and humanitarian crisis settings.
Those attending the AOFAS Annual Meeting in
1 Ly JA, Wang WL, et al. Arch Bone Jt Surg, 2022 May; 10(5): 420-425.
2 Source (SmartTrack, 2023).
About Orthofix
On January 5, 2023, Orthofix and SeaSpine merged to form a leading global spine and orthopedics company with a comprehensive portfolio of biologics, innovative spinal hardware, bone growth therapies, specialized orthopedic solutions, and a leading surgical navigation system. Its products are distributed in approximately 68 countries worldwide.
The Company is headquartered in
Forward-Looking Statements
This news release may include forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “projects,” “intends,” “predicts,” “potential,” “continue” or other comparable terminology. Orthofix cautions you that statements included in this news release that are not a description of historical facts are forward-looking statements that are based on the Company’s current expectations and assumptions. Each forward-looking statement contained in this news release is subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied by such statement. Applicable risks and uncertainties include, among others: the ability of newly launched products to perform as designed and intended and to meet the needs of surgeons and patients, including as a result of the lack of robust clinical validation; and the risks identified under the heading “Risk Factors” in Orthofix Medical Inc.’s Annual Report on Form 10-K for the fiscal year ended December 31, 2022, which was filed with the Securities and Exchange Commission (SEC) on March 6, 2023. The Company’s public filings with the Securities and Exchange Commission are available at www.sec.gov. You are cautioned not to place undue reliance on forward-looking statements, which speak only as of the date when made. Orthofix does not intend to revise or update any forward-looking statement set forth in this news release to reflect events or circumstances arising after the date hereof, except as may be required by law.
View source version on businesswire.com: https://www.businesswire.com/news/home/20230920059213/en/
Media Relations
Denise Landry
DeniseLandry@orthofix.com
214.937.2529
Investor Relations
Louisa Smith, Gilmartin Group
IR@orthofix.com
Source: Orthofix Medical Inc.







