Orthofix Announces FDA Clearance and Initial Patient Implant of the Company’s First 3D-Printed Titanium Cervical Spacer System with Nanovate Technology
Orthofix Medical Inc (NASDAQ:OFIX) has received FDA 510(k) clearance for its innovative 3D-printed CONSTRUX Mini Ti Spacer System, aimed at enhancing anterior cervical discectomy and fusion (ACDF) procedures. The first patient implant has been successfully completed. This titanium spacer incorporates Nanovate Technology, promoting bone growth and fusion. Compared to solid PEEK devices, its 3D-printed structure significantly enhances osteogenic growth factors. Orthofix aims to expand its cervical spine portfolio, with a commitment to improving patient outcomes.
- FDA 510(k) clearance received for CONSTRUX Mini Ti Spacer System.
- First patient implant successfully completed.
- 3D-printed titanium spacer designed to promote bone growth and fusion.
- Significant increase in osteogenic growth factors compared to solid PEEK devices.
- Risk of slow surgeon adoption of the CONSTRUX Mini Ti Spacer System.
- Potential for clinical data to indicate lack of improvement in patient outcomes.
- Concerns over product performance and commercial success.
- Competitors may develop superior products, impacting market position.
- Risk of insurance payers declining reimbursement for product use.
Insights
Analyzing...
Orthofix Medical Inc. (NASDAQ:OFIX), a global medical device company with a spine and extremities focus, today announced the U.S. Food and Drug Administration (FDA) 510(k) clearance and the first patient implant of the 3D-printed CONSTRUX™ Mini Ti Spacer System. Developed to enhance anterior cervical discectomy and fusion (ACDF) procedures, the CONSTRUX Mini Ti cervical spacer with Nanovate™ Technology is the first 3D-printed titanium interbody introduced to the market by Orthofix.
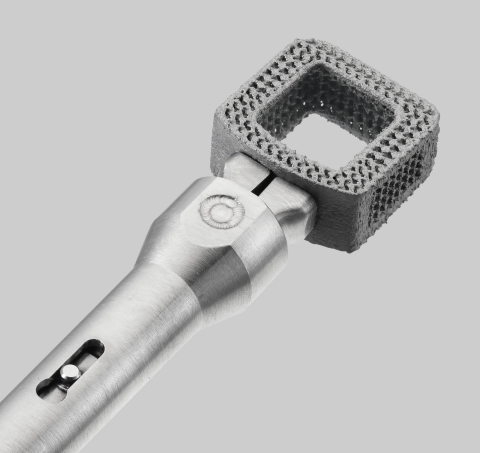
Image of Orthofix’s first 3D-printed titanium interbody, the CONSTRUX™ Mini Ti Cervical Spacer System with Nanovate™ Technology. (Photo: Orthofix Medical Inc.)
“The ACDF procedure, which is often used to treat a herniated cervical disc or degenerative disc disease, involves replacing a patient’s damaged cervical disc with an interbody packed with a biologic to promote fusion in order to provide stability and strength to the area,” said Wayne Cheng, M.D., an orthopedic spine surgeon operating at Loma Linda University Medical Center in Loma Linda, CA, who performed the first patient implant procedure. “The CONSTRUX Mini Ti System’s optimized porosity and surface allows bone to grow into and through the spacer in order to aid with the patient’s fusion.”
The CONSTRUX Mini Ti Spacer System with Nanovate Technology is one of many Orthofix products with nanotechnology FDA clearance including the CONSTRUX™ Mini PTC Spacer System, the Pillar™ SA PTC Spacer System, and the FORZA™ PTC Spacer System. When compared to solid PEEK devices, the 3D-printed endplates of these implants with Nanovate Technology show a significant increase in growth factors involved in osteogenesis and osteoblast maturation resulting in a more favorable osteogenic environment for bone ingrowth.
“Orthofix’s cervical spine offerings feature a wide array of implants ranging from motion-preserving products like the M6-C™ artificial cervical disc to advanced interbody and fixation solutions that aid surgeons in restoring spinal alignment and decreasing pain and nerve compression,” said Orthofix President of Global Spine Kevin Kenny. “We are dedicated to expanding our comprehensive cervical spine solutions with technologies like the CONSTRUX Mini Ti Spacer System that can make a meaningful difference in our product offerings and in the lives of patients.”
The CONSTRUX Mini Ti Spacer System features include:
- 3D-printed porous titanium with macro, micro, and nanoscale surface features
- Nanoscale surface that has been shown to increase proliferation and alkaline phosphatase activity (an early osteogenic differentiation marker) in human stem cells in vitro*
- Endplates with 400 micron pores and 50-percent porosity designed to help facilitate bone ingrowth**
- Functional gradient porous structure with 80-percent porosity at the midline of the implant which allows for increased fluoroscopic visualization
- Large center opening with concaved inner walls for packing bone-grafting material
- Straightforward instrumentation for easy implantation
Orthofix offers a comprehensive cervical portfolio with differentiated technologies across biologics, bone growth therapies and spinal implants. Learn more about our cervical solutions by visiting Orthofix.com.
*As suggested in an in vivo ovine lumbar spinal fusion model
**In vitro performance may not be representative of clinical performance
About Orthofix
Orthofix Medical Inc. is a global medical device and biologics company with a spine and extremities focus. The Company’s mission is to deliver innovative, quality-driven solutions as we partner with health care professionals to improve patients’ lives. Headquartered in Lewisville, Texas, Orthofix’s spine and orthopedic extremities products are distributed in more than 70 countries via the Company’s sales representatives and distributors. For more information, please visit www.Orthofix.com.
Forward-Looking Statements
This communication contains forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended, relating to our business and financial outlook, which are based on our current beliefs, assumptions, expectations, estimates, forecasts and projections. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “projects,” “intends,” “predicts,” “potential,” or “continue” or other comparable terminology. These forward-looking statements are not guarantees of our future performance and involve risks, uncertainties, estimates and assumptions that are difficult to predict, including the risks described in Part I, Item 1A under the heading Risk Factors in our Annual Report on Form 10-K for the year ended December 31, 2020 (the “2020 Form 10-K”). In addition to the risks described there, factors that could cause or contribute to such differences may include, but are not limited to: the risk that surgeons may be slow to adopt the CONSTRUX Mini Ti Spacer System; the risk that future patient studies or clinical experience and data may indicate that treatment with the CONSTRUX Mini Ti Spacer System does not improve patient outcomes as much as previously believed, or otherwise call into question the benefits of its use to patients, hospitals and surgeons; the risk that the product may not perform as intended and may therefore not achieve commercial success; the risk that competitors may develop superior products or may have a greater market position enabling more successful commercialization; the risk that insurance payers may decline to reimburse healthcare providers for the use of our products.
This list of risks, uncertainties and other factors is not complete. We discuss some of these matters more fully, as well as certain risk factors that could affect our business, financial condition, results of operations, and prospects, in reports we file from time-to-time with the SEC, which are available to read at www.sec.gov. Any or all forward-looking statements that we make may turn out to be wrong (due to inaccurate assumptions that we make or otherwise), and our actual outcomes and results may differ materially from those expressed in these forward-looking statements. You should not place undue reliance on any of these forward-looking statements. Further, any forward-looking statement speaks only as of the date hereof, unless it is specifically otherwise stated to be made as of a different date. We undertake no obligation to update, and expressly disclaim any duty to update, our forward-looking statements, whether as a result of circumstances or events that arise after the date hereof, new information, or otherwise.
View source version on businesswire.com: https://www.businesswire.com/news/home/20210405005029/en/







