NRx Pharmaceuticals (Nasdaq:NRXP) Presents Landmark Trial of NRX-101 in Suicidal Bipolar Depression At the American Society of Clinical Psychopharmacology Annual Meeting: NRX-101 is the First Oral Antidepressant Demonstrated to Reduce Suicidality in Bipolar Depression
NRx Pharmaceuticals announced the presentation of its Phase 2b/3 trial results for NRX-101 at the ASCP Annual Meeting. The study, titled 'A Randomized, Double-Blind Controlled Comparison of NRX-101 (D-cycloserine/lurasidone) to Lurasidone for Adults with Bipolar Depression and Subacute Suicidal Ideation or Behavior,' showed that NRX-101 demonstrated significant benefits over lurasidone. NRX-101 achieved a 58% reduction in the time to sustained remission from suicidality and a 76% reduction in symptoms of akathisia. No treatment-related serious adverse events were reported. The trial results suggest potential for NRX-101 as a new standard of care for bipolar depression, affecting millions globally.
- NRX-101 demonstrated a 58% reduction in time to sustained remission from suicidality compared to lurasidone.
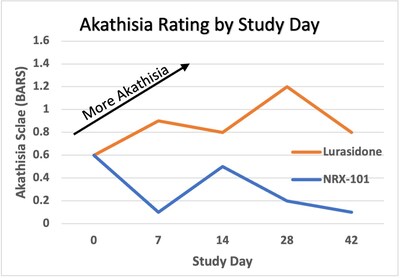
- NRX-101 showed a 76% reduction in symptoms of akathisia compared to lurasidone.
- No treatment-related serious adverse events were observed in the trial.
- The study confirms earlier findings and supports NRX-101 as a potential new standard of care for bipolar depression.
- NRX-101 may provide a significant treatment option for a large patient population (7 million in the US).
- Presentation at a highly respected conference like ASCP increases the visibility and credibility of NRX-101.
- The study observed general disorders in 18.2% of participants treated with NRX-101 compared to 0% with lurasidone.
- No difference in primary efficacy endpoint (MADRS reduction) was seen between NRX-101 and lurasidone.
- NRX-101 needs further trials for regulatory approval, which may delay its market entry.
Insights
The recent trial of NRX-101 in treating suicidal bipolar depression presented by NRx Pharmaceuticals provides compelling data on the efficacy and safety of this new treatment. NRX-101 demonstrated a 58% reduction in time to sustained remission from suicidality compared to the standard of care, lurasidone. This is significant because it addresses an urgent need within the bipolar depression patient population, where current treatments often fall short in managing suicidality. Additionally, NRX-101 showed a 76% reduction in symptoms of akathisia, a distressing side effect linked to an increased risk of suicide, compared to lurasidone.
While the efficacy results align closely with standard treatments (both showing a ~50% reduction in MADRS scores), the reduction in suicidality and akathisia positions NRX-101 as a potentially superior option for patients, especially given the serious nature of these side effects and their association with increased morbidity in bipolar patients.
However, it is important to note that these findings are derived from Phase 2b/3 trials and further validation through Phase 3 trials will be necessary for broader clinical adoption.
From a financial perspective, NRx Pharmaceuticals' recent trial results for NRX-101 could have a substantial positive impact on the company's valuation. Given the large addressable market of 7 million bipolar depression patients in the US alone, successful commercialization of NRX-101 could drive considerable revenue growth. The stock market typically reacts favorably to positive clinical trial results, particularly for conditions with significant unmet medical needs like suicidal bipolar depression.
Moreover, the potential for NRX-101 to become a standard of care treatment enhances its commercial prospects. However, investors should be cautious of the typical risks associated with clinical-stage pharmaceutical companies, such as regulatory approval hurdles, potential competition from other emerging treatments and the financial costs associated with bringing a new drug to market.
- Poster presentation of "A Randomized, Double-Blind Controlled Comparison of NRX-101 (D-cycloserine/lurasidone) to Lurasidone for Adults with Bipolar Depression and Subacute Suicidal Ideation or Behavior"
- NRX-101 demonstrated a similar antidepressant effect (MADRS reduction ~
50% ) compared to lurasidone, the Standard of Care drug - NRX-101 demonstrated a
58% relative reduction in time to sustained remission from suicidality (P=.05) compared to lurasidone - NRX-101 demonstrated a
76% reduction in symptoms of akathisia (p=0.03), a side effect linked to suicide, compared to lurasidone - This represents the second trial of NRX-101 demonstrating reduction in suicidality and akathisia associated with NRX-101 compared to lurasidone
"Presentation of these data at this highly respected conference is another important step toward bringing a life-saving product to patients in tremendous need," said Dr. Jonathan Javitt, Chairman and Chief Scientist of NRx. "We believe that NRX-101 may offer a paradigm changing approach to treatment of Bipolar Depression, with a product highly effective in both treating depression and reducing suicidality and associated side effects. We will continue working to bring hope to life with life-saving medications."
The presentation will be held at 11:15 AM, Wednesday May 29, 2024.
W89 A Randomized, Double-Blind Controlled Comparison of NRX-101 (D-cycloserine/lurasidone) to Lurasidone for Adults with Bipolar Depression and Subacute Suicidal Ideation or Behavior
CONCLUSIONS of the Poster are:
- NRX-101 and lurasidone both demonstrated >
50% response for treating bipolar depression with no difference seen on primary efficacy endpoint (MADRS) - A clinically meaningful difference was observed on both primary and secondary safety endpoints favoring NRX-101
- NRX-101 was associated with
58% relative reduction in time to sustained remission from suicidality as measured by the Columbia Suicide Severity Rating Scale (C-SSRS) when stratified by sex, mood stabilizer use, antipsychotic use, lifetime suicide event (P=0.05). - NRX-101 was associated with a relative
76% reduction in symptoms of akathisia compared to lurasidone that was sustained over 42 days (Effect Size 0.37; P=0.03) on the Barnes Akathisia Rating Scale
- NRX-101 was associated with
- Akathisia was seen in
2% of participants treated with NRX-101 vs.11% treated with lurasidone. - NRX-101 showed superiority to lurasidone in akathisia starting at day 7 and continuing through day 42/ET.
- No treatment-related serious adverse event was observed in either group. No safety issues were detected except for MedDRA General disorders: NRX-101 -
18.2% vs lurasidone -0% (p=0.002).
Based on these findings, together with the earlier STABIL-B trial, the Company believes that NRX-101 has potential to become a standard of care drug for treating bipolar depression, an addressable population of 7 million patients in the US and many times that around the world.
This study represents the second trial conducted under FDA Good Clinical Practices guidelines to demonstrate large and meaningful advantages of NRX-101 vs lurasidone on akathisia and suicidality and clears the path for a registration trial of NRX-101 vs. placebo to treat bipolar depression together with earlier accelerated approval for those with akathisia. An additional academic trial conducted by Chen and co-workers similarly demonstrated a statistically-significant reduction in suicidality associated with D-cycloserine, the active ingredient in NRX-101, compared to various standard of care antidepressants.
To the Company's knowledge, this trial and its prior STABIL-B study represent the only clinical trials in which an oral antidepressant has been demonstrated to cause meaningful reductions in suicidality and akathisia. All currently approved antidepressant drugs carry FDA-mandated "black box" warnings on their labels indicating that they may increase the risk of suicide. Similarly, akathisia – a side effect in which patients are agitated and frequently experience involuntary movement – is a side effect that occurs in 10
About NRx Pharmaceuticals
NRx Pharmaceuticals is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. The Company is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain. NRx has partnered with Alvogen and Lotus around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRx has recently announced plans to submit a New Drug Application for HTX-100 (IV ketamine), through Hope Therapeutics, in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRx was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
Notice Regarding Forward-Looking Statements
The information contained herein includes forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended. These statements include, among others, statements regarding the proposed public offering and the timing and the use of the proceeds from the offering. Forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and include words such as "may," "will," "should," "would," "expect," "plan," "believe," "intend," "look forward," and other similar expressions among others. These statements relate to future events or to the Company's future financial performance, and involve known and unknown risks, uncertainties and other factors that may cause the Company's actual results to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. You should not place undue reliance on forward-looking statements since they involve known and unknown risks, uncertainties and other factors which are, in some cases, beyond the Company's control and which could, and likely will, materially affect actual results, levels of activity, performance or achievements. Any forward-looking statement reflects the Company's current views with respect to future events and is subject to these and other risks, uncertainties and assumptions relating to the Company's operations, results of operations, growth strategy and liquidity. More detailed information about the Company and the risk factors that may affect the realization of forward-looking statements is set forth in the Company's most recent Annual Report on Form 10-K and other filings with the Securities and Exchange Commission. Investors and security holders are urged to read these documents free of charge on the SEC's website at http://www.sec.gov. Except as may be required by applicable law, The Company assumes no obligation to publicly update or revise these forward-looking statements for any reason, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, whether as a result of new information, future events or otherwise.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/nrx-pharmaceuticals-nasdaqnrxp-presents-landmark-trial-of-nrx-101-in-suicidal-bipolar-depression-at-the-american-society-of-clinical-psychopharmacology-annual-meeting-nrx-101-is-the-first-oral-antidepressant-demonstrated-to-re-302156419.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/nrx-pharmaceuticals-nasdaqnrxp-presents-landmark-trial-of-nrx-101-in-suicidal-bipolar-depression-at-the-american-society-of-clinical-psychopharmacology-annual-meeting-nrx-101-is-the-first-oral-antidepressant-demonstrated-to-re-302156419.html
SOURCE NRx Pharmaceuticals, Inc.
FAQ
What did the NRX-101 trial results show for bipolar depression?
When and where were the NRX-101 trial results presented?
What are the advantages of NRX-101 compared to lurasidone?
Were there any adverse effects noted in the NRX-101 trial?