NurExone Announces Promising Preclinical Results in Restoring Vision After Optic Nerve Damage
NurExone Biologic has announced promising results from an expanded preclinical study of ExoPTEN for optic nerve damage repair. The study, conducted with the Goldschleger Eye Institute at Sheba Medical Center, showed significant recovery in treated eyes using a rodent model of optic nerve crush.
Key findings include:
- Treated eyes regained nearly normal retinal activity
- Clear recovery of signal transmission compared to untreated controls
- Enhanced survival of retinal ganglion cells
- Successful validation through OCT scans
The Optic Nerve Disorders treatment market is projected to grow from $5.54 billion in 2023 to $11.5 billion by 2032, with a CAGR of 8.46%. The company plans to advance to a larger animal study to validate these findings.
NurExone Biologic ha annunciato risultati promettenti da uno studio preclinico ampliato su ExoPTEN per la riparazione del danno al nervo ottico. Lo studio, condotto in collaborazione con il Goldschleger Eye Institute del Sheba Medical Center, ha mostrato un recupero significativo negli occhi trattati utilizzando un modello di roditore con schiacciamento del nervo ottico.
I principali risultati includono:
- Gli occhi trattati hanno recuperato un'attività retinica quasi normale
- Chiaramente si è verificato un recupero della trasmissione del segnale rispetto ai controlli non trattati
- Aumento della sopravvivenza delle cellule gangliari retiniche
- Validazione riuscita tramite scansioni OCT
Si prevede che il mercato dei trattamenti per disturbi del nervo ottico crescerà da 5,54 miliardi di dollari nel 2023 a 11,5 miliardi di dollari entro il 2032, con un CAGR dell'8,46%. L'azienda prevede di avanzare verso uno studio su animali più grande per convalidare questi risultati.
NurExone Biologic ha anunciado resultados prometedores de un estudio preclínico ampliado sobre ExoPTEN para la reparación del daño en el nervio óptico. El estudio, realizado en colaboración con el Goldschleger Eye Institute del Sheba Medical Center, mostró una recuperación significativa en los ojos tratados utilizando un modelo de roedor de aplastamiento del nervio óptico.
Los hallazgos clave incluyen:
- Los ojos tratados recuperaron casi la actividad retiniana normal
- Recuperación clara de la transmisión de la señal en comparación con los controles no tratados
- Mayor supervivencia de las células ganglionares de la retina
- Validación exitosa a través de escaneos OCT
Se proyecta que el mercado de tratamientos para trastornos del nervio óptico crezca de 5,54 mil millones de dólares en 2023 a 11,5 mil millones de dólares para 2032, con un CAGR del 8,46%. La empresa planea avanzar a un estudio en un modelo animal más grande para validar estos hallazgos.
NurExone Biologic은 시신경 손상 수리를 위한 ExoPTEN의 확대된 전임상 연구에서 유망한 결과를 발표했습니다. 이 연구는 Sheba Medical Center의 Goldschleger Eye Institute와 협력하여 수행되었으며, 시신경 압박 모델을 사용한 실험에서 치료된 눈들이 상당한 회복을 보였습니다.
주요 발견사항은 다음과 같습니다:
- 치료된 눈들은 거의 정상적인 망막 활동을 회복했습니다
- 치료되지 않은 대조군과 비교하여 신호 전달의 명확한 회복이 나타났습니다
- 망막 신경세포의 생존율이 향상되었습니다
- OCT 스캔을 통한 성공적인 검증이 이루어졌습니다
시신경 질환 치료 시장은 2023년 55억 4천만 달러에서 2032년까지 115억 달러로 성장할 것으로 예상되며, CAGR은 8.46%입니다. 이 회사는 이러한 결과를 검증하기 위해 더 큰 동물 연구로 나아갈 계획입니다.
NurExone Biologic a annoncé des résultats prometteurs d'une étude préclinique élargie sur ExoPTEN pour la réparation des dommages au nerf optique. L'étude, menée en collaboration avec le Goldschleger Eye Institute du Sheba Medical Center, a montré une récupération significative dans les yeux traités en utilisant un modèle de rongeur de compression du nerf optique.
Les résultats clés incluent:
- Les yeux traités ont retrouvé une activité rétinienne presque normale
- Une récupération claire de la transmission du signal par rapport aux contrôles non traités
- Amélioration de la survie des cellules ganglionnaires rétiniennes
- Validation réussie par des scans OCT
Le marché des traitements des troubles du nerf optique devrait passer de 5,54 milliards de dollars en 2023 à 11,5 milliards de dollars d'ici 2032, avec un TCAC de 8,46%. L'entreprise prévoit de passer à une étude sur des animaux plus grands pour valider ces résultats.
NurExone Biologic hat vielversprechende Ergebnisse aus einer erweiterten präklinischen Studie zu ExoPTEN zur Reparatur von Schäden am Sehnerv bekannt gegeben. Die Studie, die in Zusammenarbeit mit dem Goldschleger Eye Institute im Sheba Medical Center durchgeführt wurde, zeigte eine signifikante Erholung in behandelten Augen mithilfe eines Rattenmodells für Sehnervquetschungen.
Die wichtigsten Ergebnisse umfassen:
- Behandelte Augen erlangten nahezu normale retinale Aktivität zurück
- Deutliche Wiederherstellung der Signalübertragung im Vergleich zu unbehandelten Kontrollen
- Verbesserte Überlebensrate der retinalen Ganglienzellen
- Erfolgreiche Validierung durch OCT-Scans
Der Markt für Behandlungen von Sehnervenerkrankungen wird voraussichtlich von 5,54 Milliarden Dollar im Jahr 2023 auf 11,5 Milliarden Dollar bis 2032 wachsen, mit einer CAGR von 8,46%. Das Unternehmen plant, zu einer größeren Tierversuchsstudie überzugehen, um diese Ergebnisse zu validieren.
- Successful preclinical results showing recovery of retinal activity
- Enhanced survival of retinal ganglion cells in treated eyes
- Large market opportunity with projected growth to $11.5B by 2032
- Advancement to larger animal studies for further validation
- Early-stage preclinical results requiring further validation
- Additional studies needed before potential commercialization
Paves way for additional large indications markets
TORONTO and HAIFA, Israel, Dec. 06, 2024 (GLOBE NEWSWIRE) -- NurExone Biologic Inc. (TSXV: NRX), (OTCQB: NRXBF), (Germany: J90) ("NurExone" or the "Company"), a biopharmaceutical company developing exosome-based regenerative therapies, has announced significant findings from an expanded preclinical study of the potential of its portfolio drug, ExoPTEN, for repairing optic nerve damage. Conducted in collaboration with the Goldschleger Eye Institute at Sheba Medical Center, consistently ranked one of the top ten hospitals in the world1, the study builds on previously announced preliminary results2 on June 28, 2024 and strengthens the suggestion of a promising treatment pathway for glaucoma, the leading cause of irreversible blindness globally3.
The Optic Nerve Disorders treatment market is expected to grow from 5.54 (USD Billion) in 2023 to 11.5 (USD Billion) by 2032, at a compound annual growth rate (CAGR) of ~
Researchers utilized a rodent model of optic nerve crush (ONC) to simulate the damage associated with conditions like glaucoma. After inducing injury, ExoPTEN was administrated via direct injection into the eyes. The study expanded on earlier findings which indicated that eyes treated with ExoPTEN regained nearly normal retinal activity, as evidenced by electrical tests.
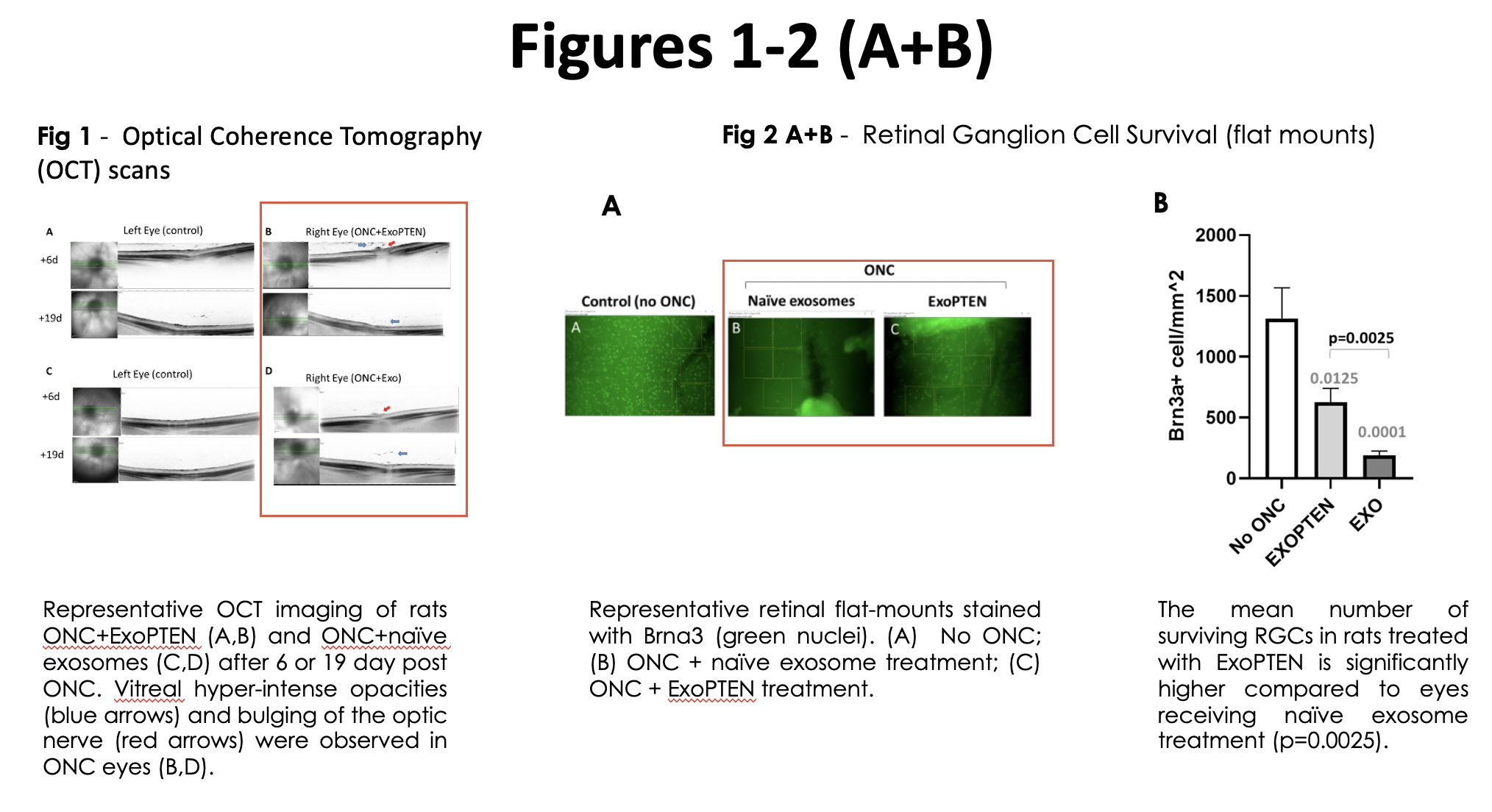
Expanded analyses of the study data showed clear recovery of signal transmission in treated eyes compared to untreated controls, which showed no significant response. Additionally, imaging results by optical coherence tomography (OCT) scans indicates and validates that in all of treated eyes (naïve exosome treatment or ExoPTEN treatment) a successful ONC procedure has been performed (Figure 1).
The study also showed that ExoPTEN treatment significantly enhanced the survival of retinal ganglion cells - key neurons responsible for transmitting visual information to the brain. Detailed analysis of retinal flat-mounts confirmed this effect, with treated eyes exhibiting substantially higher counts of these cells compared to untreated or control-treated eyes (Figures 2A and 2B).
Dr. Ifat Sher, the lead investigator from the Goldschleger Eye Institute, commented, “the results from this expanded study are extremely encouraging. ExoPTEN demonstrates potential as a treatment that restores functionality and offers neuroprotection. The study shows clear signal recovery, healthier optic nerve structures and preserved retinal ganglion cells. These results suggest that ExoPTEN could fundamentally change how we approach conditions like glaucoma and optic nerve trauma. Encouraged by these results, we are advancing to a larger study with more animals to validate and expand upon these findings.”
Dr. Lior Shaltiel, CEO of NurExone, added, “these findings are an important step forward in our mission to develop groundbreaking therapies for regenerative medicine in several indications. ExoPTEN’s ability to repair both the structure and function of the optic nerve highlights its transformative potential for addressing vision loss and improving tens of millions of patient lives.”
About NurExone
NurExone Biologic Inc. is a TSX Venture Exchange (“TSXV”) and OTCQB listed pharmaceutical company that is developing a platform for biologically guided exosome-based therapies to be delivered, minimally-invasively, to patients who have suffered Central Nervous System injuries. The Company’s first product, ExoPTEN for acute spinal cord injury, was proven to recover motor function in
For additional information and a brief interview, please watch Who is NurExone?, visit www.nurexone.com or follow NurExone on LinkedIn, X (formerly Twitter), Facebook or YouTube
Dr. Lior Shaltiel
Chief Executive Officer and Director
Phone: +972-52-4803034
Email: info@nurexone.com
Oak Hill Financial Inc.
2 Bloor Street, Suite 2900
Toronto, Ontario M4W 3E2
Investor Relations - Canada
Phone: +1-647-479-5803
Email: info@oakhillfinancial.ca
Dr. Eva Reuter
Investor Relations - Germany
Phone: +49-69-1532-5857
Email: e.reuter@dr-reuter.eu
Allele Capital Partners
Investor Relations - US
Phone: +1 978-857-5075
Email: aeriksen@allelecapital.com
FORWARD-LOOKING STATEMENTS
This press release contains certain “forward-looking statements” that reflect the Company’s current expectations and projections about its future results. Wherever possible, words such as “may”, “will”, “should”, “could”, “expect”, “plan”, “intend”, “anticipate”, “believe”, “estimate”, “predict” or “potential” or the negative or other variations of these words, or similar words or phrases, have been used to identify these forward-looking statements. Forward-looking statements in this press release include, but are not limited to, statements relating to: the results of the Company’s preclinical trials and its suggestion of a promising treatment pathway for glaucoma; the growth of the Optic Nerve Disorders treatment market; the Company expanding to further studies; the Company developing groundbreaking therapies for regenerative medicine in several indications; ExoPTEN having the potential to address vision loss and improve patient lives; and the NurExone platform technology offering novel solutions to drug companies interested in minimally invasive targeted drug delivery for other indications.
These statements reflect management’s current beliefs and are based on information currently available to management as at the date hereof. In developing the forward-looking statements in this press release, we have applied several material assumptions, including: the ability to carry out its pre-clinical trials and realize upon the stated benefits of the pre-clinical trials; the Company’s ability to realize upon the stated potential for exosome-loaded drugs in regenerating or repairing damaged nerves; the Company’s ability to maintain its ongoing commitment to using its ExoTherapy platform to advance the field of regenerative medicine; the Optic Nerve Disorders treatment market continuing to grow as stated; the Company expanding to further studies; the Company developing groundbreaking therapies for regenerative medicine in several indications; ExoPTEN addressing vision loss and improve patient lives; and the NurExone platform technology will offer novel solutions to drug companies interested in minimally invasive targeted drug delivery for other indications.
Forward-looking statements involve significant risk, uncertainties and assumptions. Many factors could cause actual results, performance or achievements to differ materially from the results discussed or implied in the forward-looking statements. These risks and uncertainties include, but are not limited to risks related to: the Company’s early stage of development; lack of revenues to date; government regulation; market acceptance for its products; rapid technological change; dependence on key personnel; dependence on the Company’s strategic partners; the fact that preclinical drug development is uncertain, and the drug product candidates of the Company may never advance to clinical trials; the fact that results of preclinical studies and early-stage clinical trials may not be predictive of the results of later stage clinical trials; the uncertain outcome, cost, and timing of product development activities, preclinical studies and clinical trials of the Company; the uncertain clinical development process, including the risk that clinical trials may not have an effective design or generate positive results; the potential inability to obtain or maintain regulatory approval of the drug product candidates of the Company; the introduction of competing drugs that are safer, more effective or less expensive than, or otherwise superior to, the drug product candidates of the Company; the initiation, conduct, and completion of preclinical studies and clinical trials may be delayed, adversely affected or impacted by unforeseen issues; the potential inability to obtain adequate financing; the potential inability to obtain or maintain intellectual property protection for the drug product candidates of the Company; risks that the Company’s intellectual property and technology won’t have the intended impact on the Company and/or its business; the Company’s inability to carry out its pre-clinical trials and realize upon the stated benefits of the pre-clinical trials; the Company’s inability to realize upon the stated potential for exosome-loaded drugs in regenerating or repairing damaged nerves; the Company’s inability to maintain its ongoing commitment to using its ExoTherapy platform to advance the field of regenerative medicine; the Optic Nerve Disorders treatment market decreasing and/or plateauing; the Company’s inability to expand into further studies; the NurExone platform technology not offering novel solutions to drug companies interested in minimally invasive targeted drug delivery for other indications; and the risks discussed under the heading “Risk Factors” on pages 44 to 51 of the Company’s Annual Information Form dated August 27, 2024, a copy of which is available under the Company’s SEDAR+ profile at www.sedarplus.ca. These factors should be considered carefully, and readers should not place undue reliance on the forward-looking statements. Although the forward-looking statements contained in this press release are based upon what management believes to be reasonable assumptions, the Company cannot assure readers that actual results will be consistent with these forward-looking statements. These forward-looking statements are made as of the date of this press release, and the Company assumes no obligation to update or revise them to reflect new events or circumstances, except as required by law.
Neither TSXV nor its Regulation Services Provider (as that term is defined in the policies of the TSXV) accepts responsibility for the adequacy or accuracy of this release.
1 https://www.afsmc.org/2024/02/sheba-medical-center-named-a-newsweek-worlds-best-hospital-for-the-6th-consecutive-year/
2 https://www.globenewswire.com/news-release/2024/06/28/2906122/0/en/NurExone-s-ExoPTEN-Being-Studied-as-Glaucoma-Treatment-for-US-3-4-Billion-Market.html
3 https://www.mdpi.com/1424-8247/17/10/1261
4 https://www.marketresearchfuture.com/reports/optic-nerve-disorders-treatment-market-30051
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/5e682a60-3287-44b2-b7da-08ebed8fa807








