NanoVibronix to Launch Rental Program for PainShield and UroShield
NanoVibronix, Inc. (NASDAQ: NAOV) has announced the launch of a month-to-month rental program for its medical devices, PainShield and UroShield, effective March 1, 2023. This initiative aims to improve patient accessibility and convenience, allowing individuals with valid prescriptions to rent these devices at affordable rates. The CEO, Brian Murphy, expressed confidence that the program will qualify for Medicare reimbursement, with additional supplies available for purchase separately. The company has applied for Medicare reimbursement, expecting a decision by May 2023 and implementation by October 1, 2023.
- Launch of month-to-month rental program for PainShield and UroShield on March 1, 2023, increasing patient accessibility.
- Confidence in Medicare reimbursement eligibility for the rental program, enhancing potential revenue streams.
- Company aims to expand its product offerings to meet patient needs.
- Pending Medicare reimbursement decision in May 2023 could impact product availability and sales.
- Uncertainties related to market acceptance and reimbursement from third-party payers may pose revenue risks.
Program Will Bring Greater Convenience and Accessibility to Patients
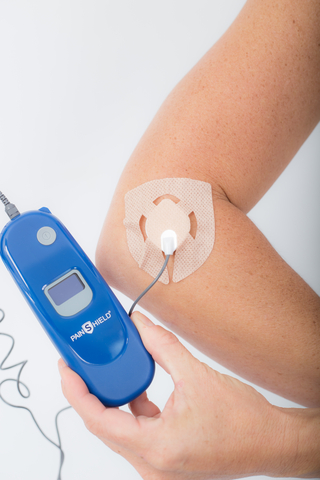
PainShield by
“Patients with a valid prescription will soon be able to rent PainShield and UroShield for use in their own home on a month-to-month basis, at affordable prices, and with little ongoing commitment,” stated
“We are extremely excited to be able to expand our product offering to meet patient needs,” continued Murphy. “This program is another example of our stated goal to develop sustainable and portable alternatives to medication, pain relief drugs, and medical device care.”
The Company recently applied for Medicare reimbursement. We anticipate a decision on the application in
About
Forward-looking Statements
This press release contains “forward-looking statements.” Such statements may be preceded by the words “intends,” “may,” “will,” “plans,” “expects,” “anticipates,” “projects,” “predicts,” “estimates,” “aims,” “believes,” “hopes,” “potential” or similar words. Forward-looking statements are not guarantees of future performance, are based on certain assumptions and are subject to various known and unknown risks and uncertainties, many of which are beyond the Company’s control, and cannot be predicted or quantified; consequently, actual results may differ materially from those expressed or implied by such forward-looking statements. Such risks and uncertainties include, without limitation, risks and uncertainties associated with: (i) market acceptance of our existing and new products or lengthy product delays in key markets; (ii) negative or unreliable clinical trial results; (iii) inability to secure regulatory approvals for the sale of our products; (iv) intense competition in the medical device industry from much larger, multinational companies; (v) product liability claims; (vi) product malfunctions; (vii) our limited manufacturing capabilities and reliance on subcontractor assistance; (viii) insufficient or inadequate reimbursements by governmental and/or other third party payers for our products; (ix) our ability to successfully obtain and maintain intellectual property protection covering our products; (x) legislative or regulatory reform impacting the healthcare system in the
View source version on businesswire.com: https://www.businesswire.com/news/home/20230222005407/en/
Investor Contacts:
brett@haydenir.com
(646) 536-7331
Source:
FAQ
What is the new rental program for NanoVibronix's products?
When will the Medicare reimbursement application for NanoVibronix be decided?
What are the benefits of the PainShield and UroShield rental program?







