NanoVibronix Extends Distribution Agreement with Its Largest Distributor for PainShield and PainShield Plus
- UPPI is the largest distributor of PainShield and PainShield Plus, indicating strong demand for the products.
- The agreement includes immediate restocking orders and minimum purchase guarantees, providing stability for NanoVibronix.
- Efforts to obtain approval from CMS for reimbursements are ongoing, with a favorable outcome expected.
- Approval from CMS could lead to reimbursements starting in October 2023, creating additional opportunities for NanoVibronix.
- None.
Extension Provides for Guaranteed Purchase Minimums
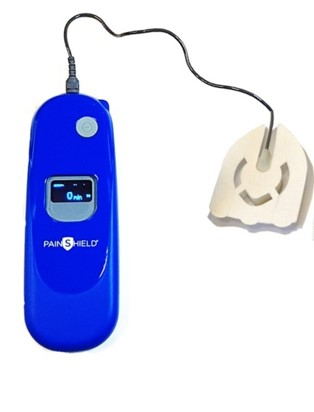
Wearable Ultrasound-PainShield MD (Photo: Business Wire)
Under the terms of the extended agreement, UPPI will continue to be the exclusive distributor of PainShield and PainShield Plus devices to the Durable Medical Equipment distribution sector of the healthcare market in
Brian Murphy, Chief Executive Officer of NanoVibronix, Inc., commented, “Since executing the original distribution agreement in 2020, UPPI has grown to be our largest distributor of PainShield and PainShield Plus. The team at UPPI understands the importance of providing clinicians and patients with non-narcotic/non-opiate clinincally-supported modalities to reduce the economic burden on the healthcare system and limit the amount of lost working days for injured workers,1 which makes them a valuable channel for our products.
“Our efforts to obtain full approval from the Centers for Medicare & Medicaid Services (‘CMS’) continue. We submitted the final report with our application to CMS in March 2023, and we remain hopeful for a favorable outcome. If approved, reimbursements could begin as early as October 1 of this year. The new agreement with UPPI takes into consideration approval from CMS and provides for modifications that consider the opportunities afforded through reimbursement.”
About NanoVibronix, Inc.
NanoVibronix, Inc. (NASDAQ: NAOV) is a medical device company headquartered in
Forward-looking Statements
This press release contains “forward-looking statements.” Such statements may be preceded by the words “intends,” “may,” “will,” “plans,” “expects,” “anticipates,” “projects,” “predicts,” “estimates,” “aims,” “believes,” “hopes,” “potential” or similar words. Forward-looking statements are not guarantees of future performance, are based on certain assumptions and are subject to various known and unknown risks and uncertainties, many of which are beyond the Company’s control, and cannot be predicted or quantified; consequently, actual results may differ materially from those expressed or implied by such forward-looking statements. Such risks and uncertainties include, without limitation, risks and uncertainties associated with: (i) market acceptance of our existing and new products or lengthy product delays in key markets; (ii) negative or unreliable clinical trial results; (iii) inability to secure regulatory approvals for the sale of our products; (iv) intense competition in the medical device industry from much larger, multinational companies; (v) product liability claims; (vi) product malfunctions; (vii) our limited manufacturing capabilities and reliance on subcontractor assistance; (viii) insufficient or inadequate reimbursements by governmental and/or other third party payers for our products; (ix) our ability to successfully obtain and maintain intellectual property protection covering our products; (x) legislative or regulatory reform impacting the healthcare system in the
______________________________
1 https://ultrapainpro.com/about-us/
View source version on businesswire.com: https://www.businesswire.com/news/home/20230823426842/en/
Investor Contact:
Brett Maas, Managing Principal, Hayden IR, LLC
brett@haydenir.com
(646) 536-7331
Source: NanoVibronix, Inc.







