Mylan Initiates Voluntary Nationwide Recall of Four Lots of Amiodarone HCl Injection, USP and Tranexamic Acid Injection, USP Due to Carton Label Mix-Up
Mylan N.V. (NASDAQ: MYL) has announced a voluntary nationwide recall of four lots of Amiodarone HCl Injection and Tranexamic Acid Injection due to critical carton labeling errors. These products, distributed to wholesalers and hospital pharmacies, could contain mislabeled vials, posing severe risks to patient safety. Both medications are only to be used by trained healthcare professionals. Mylan has reported no adverse events linked to this recall to date. The company is notifying distributors and arranging product returns.
- No adverse events reported related to the recall.
- Potential risk to patient safety due to labeling mix-up.
- Recall may impact brand reputation and sales.
Insights
Analyzing...
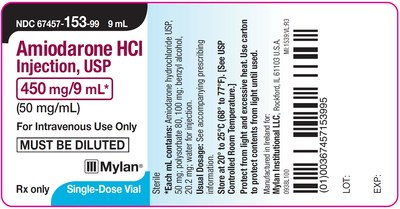
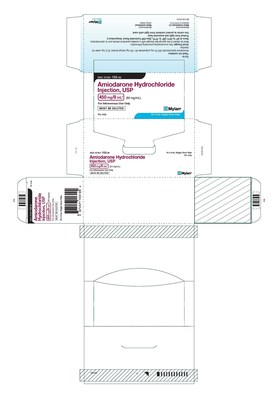
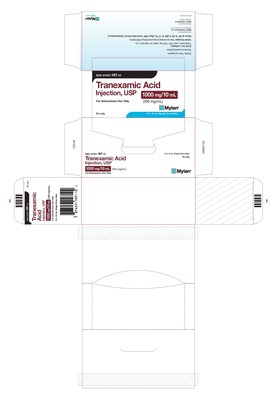
HERTFORDSHIRE, England and PITTSBURGH, Aug. 28, 2020 /PRNewswire/ -- Mylan N.V. (NASDAQ: MYL) today announced that its U.S.-based Mylan Institutional LLC business is conducting a voluntary nationwide recall to the hospital/clinic level of four lots of Amiodarone HCl Injection, USP 450 mg/9 mL, packaged in cartons of 10 single-dose 9 mL vials and Tranexamic Acid Injection, USP 1000 mg/10 mL, packaged in cartons of 10 single-dose 10 mL vials.
These batches are being recalled due to the potential for cartons labeled as Tranexamic Acid Injection, USP to contain vials of Amiodarone HCl Injection, USP and cartons labeled as Amiodarone HCl Injection, USP to contain vials of Tranexamic Acid Injection, USP. The individual vials contained within the cartons are accurately labeled as Amiodarone HCl Injection, USP or Tranexamic Acid Injection, USP. Both of these medications are administered in a hospital setting only by trained healthcare professionals. To date, Mylan has not received any reports of adverse events related to this recall.
Amiodarone HCl Injection, USP and Tranexamic Acid Injection, USP are used to treat different conditions. If Tranexamic acid is administered to a patient in place of Amiodarone or vice versa, it could present a risk to patient safety. If Amiodarone HCl Injection is inadvertently administered it could result in low blood pressure and irregular heartbeat, including lower than expected heart rate, which could have immediate life-threatening effects on cardiac function. If treatment with Amiodarone HCl Injection, when needed, is delayed this could result in continued irregular heartbeat and potential life-threatening effects on cardiac function. If Tranexamic Acid Injection is inadvertently administered it could result in adverse events, including blood clotting, seizures, hypersensitivity reactions, visual disturbances, and dizziness. If treatment with Tranexamic Acid Injection, when needed, is delayed this could result in limited to serious and life-threatening bleeding events.
Amiodarone HCl Injection, USP is an antiarrhythmic agent indicated for initiation of treatment and prophylaxis of frequently recurring ventricular fibrillation (VF) and hemodynamically unstable ventricular tachycardia (VT) in patients' refractory to other therapy. Tranexamic acid injection is indicated in patients with hemophilia for short term use to reduce or prevent hemorrhage and reduce the need for replacement therapy during and following tooth extraction.
These batches were distributed nationwide in the USA to wholesalers and hospital/clinical pharmacies between April 2020 and July 2020. The recalled batch information is as follows:
NDC # | Material Description | Strength | Carton Size | Lot No. | Expiry |
67457-153-09 | Amiodarone HCl Injection, USP | 450 mg/9 mL | 10 x 9 mL single-dose vials | 191207 191221 191223 200120 | Nov. 2021 Nov. 2021 Nov. 2021 Dec. 2021 |
67457-197-10 | Tranexamic Acid Injection, USP | 1000 mg/10 mL | 10 x 10 mL single-dose vials |
Mylan is notifying its wholesalers and hospital/clinic pharmacies by letter and is arranging for return of recalled products to Stericycle. Wholesalers and hospital/clinic pharmacies that have product which is being recalled should stop use/further distribution or dispensing. Wholesalers and hospital/clinic pharmacies that are in possession of recalled product should contact Stericycle at 1-888-410-7505 for the return of the recalled product. Normal business hours are Monday through Friday 8 a.m. to 5 p.m. EST.
Consumers with questions regarding this recall can contact Mylan Customer Relations at 800.796.9526 or customer.service@mylan.com, Monday through Friday from 8 a.m. – 5 p.m. EST. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to using these drug products.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form or submit by fax to 1-800-FDA-0178.
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
About Mylan
Mylan is a global pharmaceutical company committed to setting new standards in healthcare. Working together around the world to provide 7 billion people access to high quality medicine, we innovate to satisfy unmet needs; make reliability and service excellence a habit; do what's right, not what's easy; and impact the future through passionate global leadership. We offer a portfolio of more than 7,500 marketed products around the world, including antiretroviral therapies on which approximately
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/mylan-initiates-voluntary-nationwide-recall-of-four-lots-of-amiodarone-hcl-injection-usp-and-tranexamic-acid-injection-usp-due-to-carton-label-mix-up-301120584.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/mylan-initiates-voluntary-nationwide-recall-of-four-lots-of-amiodarone-hcl-injection-usp-and-tranexamic-acid-injection-usp-due-to-carton-label-mix-up-301120584.html
SOURCE Mylan N.V.