Monopar Announces Positive Preclinical Therapeutic Isotope Data for its MNPR-101 Radiopharma Program
- None.
- None.
Insights
The recent preclinical imaging data from Monopar Therapeutics Inc. indicates a significant advancement in the field of targeted cancer therapy. The proprietary uPAR targeting agent MNPR-101, when bound to therapeutic radioisotopes, has demonstrated a highly preferential uptake in tumors. This specificity is crucial in the context of radiopharmaceuticals because it could potentially minimize damage to healthy tissue and enhance the therapeutic index.
Comparing Monopar's approach to existing radiopharmaceuticals like Pluvicto® and Lutathera™, which utilize Lutetium-177, Monopar's MNPR-101 conjugated to Lu-177 shows promise in achieving similar or improved efficacy. The ability to visualize biodistribution via SPECT imaging is a significant advantage, allowing for real-time monitoring of the treatment's progress and facilitating personalized treatment adjustments.
The implications of these findings are far-reaching for patients with uPAR-positive cancers, which include aggressive forms like triple-negative breast cancer and pancreatic cancer. The data bolsters Monopar's strategic move towards a Phase 1 dosimetry clinical trial, which is a critical step in the drug development process. If successful in clinical trials, Monopar's technology could lead to new treatment options for patients with limited alternatives, potentially improving outcomes and survival rates.
The uPAR system is a well-known target in oncology due to its role in tumor invasion, metastasis and angiogenesis. Monopar's MNPR-101's ability to target uPAR and deliver radioisotopes directly to the tumor site is a promising therapeutic strategy, especially for cancers that are notoriously difficult to treat. The reported near-complete elimination of tumors in preclinical models after a single injection is particularly noteworthy, suggesting that this approach could offer a potent and less frequent treatment regimen, which would be a significant improvement in patient quality of life compared to current therapies.
As an oncologist, the potential to pair diagnostic imaging with therapeutic intervention using the same molecular targeting agent is exciting. This could enable a more precise and tailored approach to cancer therapy, improving the selection of patients who are most likely to benefit from MNPR-101 based treatments. The upcoming Phase 1 dosimetry clinical trial in Australia will be an important milestone to assess the safety and optimal dosing of MNPR-101-Zr in humans, which is a precursor to evaluating therapeutic efficacy.
From a market perspective, the development of targeted radiopharmaceuticals represents a growing niche within the broader oncology market. Monopar Therapeutics' positive preclinical data positions them favorably within this space, especially given the high market demand for effective treatments for aggressive cancers such as triple-negative breast cancer and pancreatic cancer. The company's progress also signals potential interest from larger pharmaceutical companies looking for promising candidates to expand their oncology pipelines through partnerships or acquisitions.
Investors should note that while the preclinical results are promising, the transition to clinical success is not guaranteed. The upcoming Phase 1 trial will be closely monitored as it will provide insights into human safety and dosimetry, which are critical for regulatory approval and eventual commercialization. Positive outcomes from the trial could lead to increased investor confidence and potentially drive up Monopar's market valuation.
WILMETTE, Ill., March 05, 2024 (GLOBE NEWSWIRE) -- Monopar Therapeutics Inc. (Nasdaq: MNPR), a clinical-stage biopharmaceutical company focused on developing innovative treatments for cancer patients, today announced positive preclinical imaging data of a therapeutic radioisotope bound to its proprietary uPAR (urokinase plasminogen activator receptor) targeting agent MNPR-101. The data clearly demonstrate highly preferential uptake in the tumor. This is an extension of the tumor imaging and efficacy data Monopar released on February 22 (link), where Monopar disclosed biodistribution data with a diagnostic imaging radioisotope (Zirconium-89) as well as efficacy data with therapeutic radioisotopes (e.g., Actinium-225) bound to MNPR-101 in human tumor xenograft models. The new imaging data released today provide additional support for the tumor-targeting ability of MNPR-101 and help explain the near complete elimination of tumors (link) observed after a single injection of therapeutic radioisotopes bound to MNPR-101.
Biodistribution of a Therapeutic Radioisotope Conjugated to MNPR-101
Two of the most commercially successful radiopharmaceuticals, Pluvicto® and Lutathera™, use the therapeutic radioisotope Lutetium-177 (Lu-177). Beyond killing cancer cells, this radioisotope has the added advantage that its biodistribution can be visualized via SPECT (single-photon emission computed tomography) imaging. Monopar collected a sequential SPECT imaging time-series utilizing MNPR-101 conjugated to Lu-177 (MNPR-101-Lu) in a uPAR-expressing human pancreatic cancer xenograft model. The results can be seen in Figure 1. High specificity and durable uptake of MNPR-101-Lu in the tumor relative to normal tissue is readily apparent, and these results are consistent with the previously released data for Monopar’s diagnostic imaging radiopharmaceutical MNPR-101-Zr.
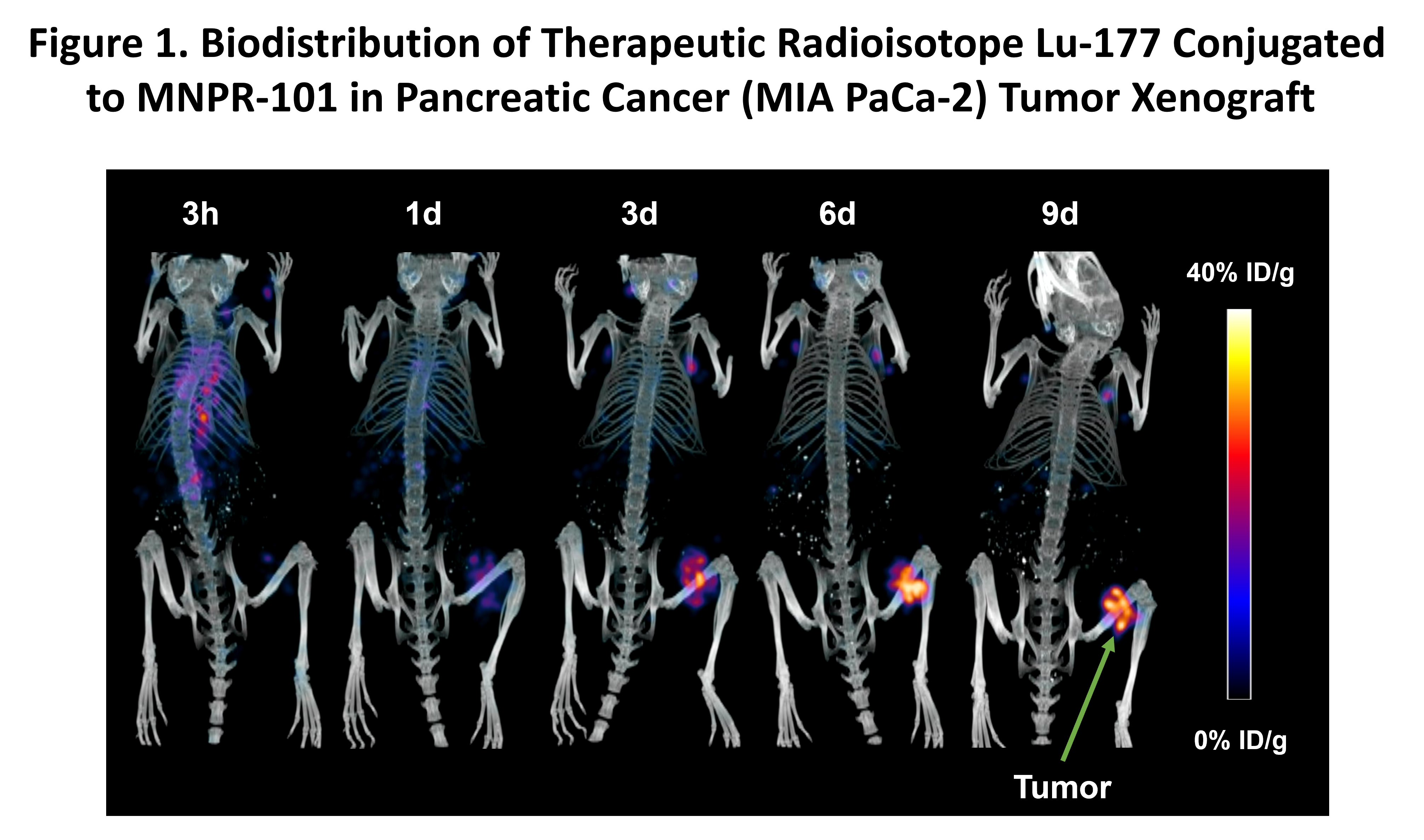
“Delivering a high dose to the tumor relative to normal tissue is of central importance in radiopharmaceutical therapy,” said Andrew Cittadine, Monopar’s Chief Operating Officer. “These data help explain the compelling and durable anti-tumor benefits observed to-date in preclinical studies using MNPR-101 conjugated to therapeutic radioisotopes.”
Monopar recently announced it received Human Research Ethics Committee (HREC) clearance in Australia to commence a Phase 1 dosimetry clinical trial for MNPR-101-Zr in advanced cancer patients (link). The data disclosed today further support Monopar’s efforts to create a radiodiagnostic and radiotherapeutic pairing to image and treat uPAR-positive cancers.
“Some of the most aggressive, deadly cancers express uPAR, such as triple negative breast cancer and pancreatic cancer,” said Chandler Robinson, MD, Monopar’s Chief Executive Officer. “These data further support the potential of a MNPR-101 based radiopharmaceutical to provide a very meaningful clinical benefit to patients with uPAR-positive tumors.”
About Monopar Therapeutics Inc.
Monopar Therapeutics is a clinical-stage biopharmaceutical company focused on developing innovative treatments for cancer patients. Monopar's pipeline consists of Phase 1b-stage camsirubicin for the treatment of advanced soft tissue sarcoma; Phase 1-stage MNPR-101 for radiopharmaceutical use in various advanced cancers; and an early-stage camsirubicin analog, MNPR-202. For more information, visit: www.monopartx.com.
Forward-Looking Statements
Statements contained in this press release regarding matters that are not historical facts are "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. The words “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “target” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Examples of these forward-looking statements include: that data disclosed today further support Monopar’s efforts to create a radiodiagnostic and radiotherapeutic pairing to image and treat uPAR-positive cancers; and that these data further support the potential of a MNPR-101 based radiopharmaceutical to provide a very meaningful clinical benefit to patients with uPAR-positive tumors. The forward-looking statements involve risks and uncertainties including, but not limited to: that future preclinical or clinical data will not be as promising as the data to date; not initiating and enrolling the Phase 1 clinical trial; that MNPR-101-Zr and/or MNPR-101-Lu may cause unexpected serious adverse effects or fail to image or be effective against the cancer tumors in humans; the potential for the HREC to put the Phase 1 trial on clinical hold at any time; and the significant general risks and uncertainties surrounding the research, development, regulatory approval, and commercialization of imaging agents and therapeutics. Actual results may differ materially from those expressed or implied by such forward-looking statements. Risks are described more fully in Monopar's filings with the Securities and Exchange Commission. All forward-looking statements contained in this press release speak only as of the date on which they were made. Monopar undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made. Any forward-looking statements contained in this press release represent Monopar’s views only as of the date hereof and should not be relied upon as representing its views as of any subsequent date.
CONTACT:
Monopar Therapeutics Inc.
Investor Relations
Kim R. Tsuchimoto
Chief Financial Officer
kimtsu@monopartx.com
Follow Monopar on social media for updates:
Twitter: @MonoparTx LinkedIn: Monopar Therapeutics
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/0773bb83-f9ca-4ee3-b947-37260033b8a9

FAQ
What is the ticker symbol for Monopar Therapeutics Inc.?
What did Monopar announce regarding preclinical imaging data?
What clearance did Monopar receive recently?
Which cancers express uPAR?







