3M V.A.C. Therapy negative pressure wound therapy achieves key medical evidence milestone, surpassing 2,000 peer-reviewed medical journal studies published
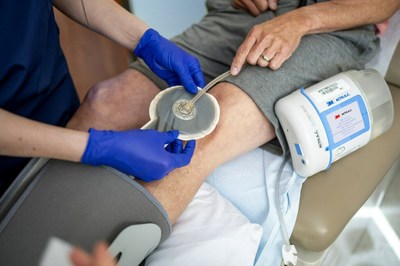
3M Health Care's Medical Solutions Division announced that its 3M™ V.A.C.® Therapy negative pressure wound therapy (NPWT) has surpassed a significant clinical milestone with over 2,000 peer-reviewed studies published. This achievement highlights V.A.C. Therapy as the most clinically supported NPWT solution, accounting for 75% of all published NPWT evidence. The studies cover a variety of wound types and care settings, promoting innovations in wound management. With continuous product evolution, 3M focuses on enhancing patient outcomes and clinician efficiency in wound care.
- Achieved milestone of 2,000 published, peer-reviewed studies for V.A.C. Therapy.
- Holds over 75% of published NPWT clinical evidence.
- Continuous product innovations, including the launch of the first silicone-acrylic hybrid drape.
- None.
Insights
Analyzing...
ST. PAUL, Minn., Oct. 13, 2022 /PRNewswire/ -- 3M Health Care's Medical Solutions Division today announced its 3M™ V.A.C.® Therapy negative pressure wound therapy (NPWT) has surpassed a clinical evidence milestone of 2,000 published, peer-reviewed medical journal studies. V.A.C. Therapy is the first and only NPWT solution to garner this number of published studies about its therapy. It is backed by more clinical data than any other brand, accounting for more than
The clinical studies have been conducted by wound care professionals worldwide and published in journals across the globe, covering a comprehensive range of wound types, wound care settings and study formats, such as case studies, economic studies, randomized controlled trials and more.
"Clinical evidence has always been a foundational element to establishing credibility for V.A.C. Therapy and our NPWT products in the wound care community," said Ronald Silverman, M.D., 3M Health Care senior vice president of clinical affairs and chief medical officer. "Published studies have also helped to promote adoption of NPWT and spur therapy innovations, including 3M™ Prevena™ Therapy for incision management, 3M™ Veraflo™ Therapy for instillation therapy for open wounds, 3M™ AbThera™ Open Abdomen Negative Pressure Therapy. Our team members in the field are also actively engaged with wound care experts worldwide, working right alongside clinicians to observe the changing nature of wound care and gather feedback about our products, which helps us identify opportunities for innovation."
Today, V.A.C. Therapy is used across a spectrum of health care settings, from acute care facilities to ambulatory surgical centers, assisted living facilities, and in patients' homes. In the U.S., V.A.C. Therapy is available with 24/7 remote therapy monitoring to support adherence to the therapy. 3M's NPWT portfolio continuously evolves to meet clinician and patient needs. Last year, 3M launched the first-ever silicone-acrylic hybrid drape for use with V.A.C. Therapy, the 3M™ Dermatac™ Drape, an innovation designed to be gentle on patients' skin and easy for clinicians to use.
"Today's wound care patients are often sicker and have more comorbidities, making their wounds more complex to treat and increasing the demands on clinicians' time. 3M strives to provide a robust tool selection to address clinicians' unique wound care needs and make our products easier to use to help save their valuable time -- and ultimately, help transform outcomes and improve lives for wound care patients," said Dr. Silverman.
For more information, visit www.3m.com/npwt.
About 3M
3M (NYSE: MMM) believes science helps create a brighter world for everyone. By unlocking the power of people, ideas and science to reimagine what's possible, our global team uniquely addresses the opportunities and challenges of our customers, communities, and planet. Learn how we're working to improve lives and make what's next at 3M.com/news or on Twitter at @3M or @3MNews.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/3m-vac-therapy-negative-pressure-wound-therapy-achieves-key-medical-evidence-milestone-surpassing-2-000-peer-reviewed-medical-journal-studies-published-301648149.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/3m-vac-therapy-negative-pressure-wound-therapy-achieves-key-medical-evidence-milestone-surpassing-2-000-peer-reviewed-medical-journal-studies-published-301648149.html
SOURCE 3M