Moleculin Announces Plans for MIRACLE Phase 3 Pivotal Trial
Moleculin Biotech (Nasdaq: MBRX) has announced plans for its MIRACLE Phase 3 pivotal trial following a positive End of Phase 1B/2 meeting with the FDA. The trial will evaluate Annamycin in combination with cytarabine (AnnAraC) for treating relapsed or refractory acute myeloid leukemia (R/R AML). Key points include:
1) The trial will be global, including US sites.
2) It will use an adaptive design with CR (complete remission) at day 30 as the primary endpoint.
3) The study will allow dosing above the lifetime maximum allowable anthracycline dose.
4) The trial design offers potential for accelerated approval.
5) Initial focus on 2nd line R/R AML treatment, followed by 3rd line R/R AML.
Moleculin aims to begin contracting trial sites in 2H 2024, with the first subject treated in Q1 2025. The company expects to submit an NDA for accelerated approval in 2H 2028.
Moleculin Biotech (Nasdaq: MBRX) ha annunciato i piani per il suo trial clinico pivotale MIRACLE di fase 3 dopo un incontro positivo di fine fase 1B/2 con la FDA. Il trial valuterà Annamycin in combinazione con cytarabine (AnnAraC) per il trattamento della leucemia mieloide acuta in recidiva o refrattaria (R/R AML). I punti chiave includono:
1) Il trial sarà globale, includendo siti negli Stati Uniti.
2) Utilizzerà un design adattivo con CR (remissione completa) al giorno 30 come endpoint primario.
3) Lo studio permetterà dosi superiori alla dose massima consentita di antracicline nel corso della vita.
4) Il design del trial offre la possibilità di approvazione accelerata.
5) Iniziale focus sul trattamento di 2a linea per R/R AML, seguito dal trattamento di 3a linea per R/R AML.
Moleculin prevede di iniziare a contrattare i siti del trial nel secondo semestre del 2024, con il primo soggetto trattato nel primo trimestre del 2025. L'azienda si aspetta di presentare una NDA per approvazione accelerata nel secondo semestre del 2028.
Moleculin Biotech (Nasdaq: MBRX) ha anunciado planes para su ensayo clínico pivotal MIRACLE de fase 3 tras una reunión positiva de fin de fase 1B/2 con la FDA. El ensayo evaluará Annamycin en combinación con citarabina (AnnAraC) para tratar la leucemia mieloide aguda en recaída o refractaria (R/R AML). Los puntos clave incluyen:
1) El ensayo será global, incluyendo sitios en EE. UU.
2) Utilizará un diseño adaptativo con CR (remisión completa) al día 30 como el objetivo primario.
3) El estudio permitirá dosis superiores a la máxima dosis de antraciclinas permitida durante la vida.
4) El diseño del ensayo ofrece la posibilidad de aprobación acelerada.
5) Enfoque inicial en el tratamiento de 2da línea para R/R AML, seguido por el tratamiento de 3ra línea para R/R AML.
Moleculin espera comenzar a contratar sitios para el ensayo en el segundo semestre de 2024, con el primer sujeto tratado en el primer trimestre de 2025. La empresa espera presentar una NDA para aprobación acelerada en el segundo semestre de 2028.
Moleculin Biotech (Nasdaq: MBRX)는 FDA와의 긍정적인 1B/2단계 회의 후, MIRACLE 3상 주요 임상시험 계획을 발표했습니다. 본 시험은 재발성 또는 불응성 급성 골수성 백혈병(R/R AML) 치료를 위해 안나마이신과 사이타라빈(AnnAraC)의 병용 요법을 평가할 것입니다. 주요 사항은 다음과 같습니다:
1) 시험은 미국 사이트를 포함한 글로벌 방식으로 진행됩니다.
2) 30일째 완전 관해(CR)를 기본 endpoint로 하는 적응형 설계를 사용할 것입니다.
3) 연구는 최대 평생 안트라사이클린 용량을 초과하는 투약을 허용합니다.
4) 시험 설계는 신속 승인 가능성을 제공합니다.
5) 처음에는 2차 R/R AML 치료에 중점을 두고, 이어서 3차 R/R AML 치료를 진행합니다.
Moleculin은 2024년 하반기에 시험 사이트 계약을 시작하고, 2025년 1분기에 첫 번째 피험자를 치료할 계획입니다. 회사는 2028년 하반기에 신속 승인 신청(NDA)을 제출할 것으로 예상하고 있습니다.
Moleculin Biotech (Nasdaq: MBRX) a annoncé ses plans pour son essai clinique pivot MIRACLE de phase 3 suite à une réunion positive de fin de phase 1B/2 avec la FDA. L'essai évaluera Annamycin en combinaison avec la cytarabine (AnnAraC) pour le traitement de la leucémie myéloïde aiguë en rechute ou réfractaire (R/R AML). Les points clés incluent :
1) L'essai sera mondial, incluant des sites aux États-Unis.
2) Il utilisera un design adaptatif avec CR (rémission complète) au jour 30 comme critère principal.
3) L'étude autorisera des doses supérieures à la dose maximale autorisée d'anthracyclines au cours de la vie.
4) Le design de l'essai offre un potentiel pour une approbation accélérée.
5) Mise au point initiale sur le traitement de 2e ligne pour R/R AML, suivie par le traitement de 3e ligne pour R/R AML.
Moleculin prévoit de commencer à contractualiser des sites d'essai au second semestre 2024, avec le premier sujet traité au premier trimestre 2025. L'entreprise s'attend à soumettre une NDA pour une approbation accélérée au second semestre 2028.
Moleculin Biotech (Nasdaq: MBRX) hat Pläne für seine MIRACLE Phase-3-Studie bekannt gegeben, nachdem es ein positives Ende der Phase-1B/2-Besprechung mit der FDA gab. Die Studie wird Annamycin in Kombination mit Cytarabin (AnnAraC) zur Behandlung von rückfälliger oder refraktärer akuter myeloischer Leukämie (R/R AML) evaluieren. Die wichtigsten Punkte sind:
1) Die Studie wird global sein, einschließlich Standorte in den USA.
2) Sie wird ein adaptives Design verwenden, wobei CR (vollständige Remission) am Tag 30 als primärer Endpunkt dient.
3) Die Studie erlaubt Dosen, die über der maximal zulässigen Lebensdosis von Antrazeen liegen.
4) Das Studiendesign bietet Potenzial für eine beschleunigte Genehmigung.
5) Zunächst Fokus auf die Behandlung von 2. Linie R/R AML, gefolgt von der 3. Linie R/R AML.
Moleculin plant, in der zweiten Hälfte von 2024 mit der Vertragsverhandlungen für Studienstandorte zu beginnen, wobei der erste Proband im ersten Quartal 2025 behandelt werden soll. Das Unternehmen erwartet, in der zweiten Hälfte von 2028 einen NDA zur beschleunigten Genehmigung einzureichen.
- FDA supports advancement to Phase 3 pivotal trial for Annamycin in R/R AML treatment
- Potential for accelerated approval based on CR at day 30 as primary endpoint
- Adaptive trial design allows for optimization of Annamycin dosing
- Global trial including US sites, expanding market potential
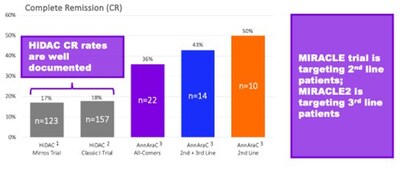
- AnnAraC has demonstrated more than double the CR rate compared to historical HiDAC data
- Annamycin has Fast Track Status and Orphan Drug Designation from FDA and EMA
- Final data for 2nd line subjects not expected until 2027
- NDA submission for accelerated approval not planned until 2H 2028
- Potential risks associated with dosing above lifetime maximum allowable anthracycline dose
Insights
The announcement of Moleculin's plans for the MIRACLE Phase 3 pivotal trial for Annamycin in combination with cytarabine (AnnAraC) is a significant development in the treatment of relapsed or refractory acute myeloid leukemia (R/R AML). This trial design, based on discussions with the FDA, presents several key points of interest:
- The primary endpoint of complete remission (CR) at day 30 versus placebo offers a clear, measurable outcome that could lead to accelerated approval if met.
- The adaptive design, with an initial cohort of 75 patients to determine the optimal dose, demonstrates a strategic approach to drug development that balances efficacy and safety.
- The ability to dose above the lifetime maximum allowable anthracycline dose in the US is noteworthy, as it may allow for more effective treatment regimens.
From a clinical perspective, the historical data showing AnnAraC's CR rate at more than double that of high-dose cytarabine (HiDAC) alone is particularly promising. If replicated in the Phase 3 trial, this could represent a significant advancement in R/R AML treatment options.
The inclusion of durability of response (DoR) and overall survival (OS) as secondary endpoints will provide important long-term efficacy data. Additionally, the planned MIRACLE2 trial for 3rd line patients will offer insights into the treatment's efficacy in more heavily pretreated populations.
While the timeline extending to 2028 for potential NDA submission may seem lengthy, it's important to note that this is not unusual for pivotal oncology trials, especially those targeting rare diseases like AML.
Moleculin's announcement of its Phase 3 MIRACLE trial plans represents a significant milestone for the company and potentially for AML treatment. Several aspects of this development are particularly noteworthy from an industry perspective:
- The FDA's apparent receptiveness to the trial design, including the use of CR at day 30 as the primary endpoint for potential accelerated approval, suggests a favorable regulatory environment for this approach.
- The adaptive trial design, allowing for dose optimization mid-study, aligns with the FDA's Project Optimus initiative, demonstrating Moleculin's alignment with current regulatory trends.
- The global nature of the trial, including US sites, broadens the potential market for Annamycin and may facilitate future approvals in multiple regions.
Financially, the progression to Phase 3 typically represents a value inflection point for biotech companies. However, investors should note that the extended timeline to potential NDA submission in 2H 2028 implies significant ongoing development costs.
The market potential for a successful R/R AML treatment is substantial, given the poor prognosis for these patients. If Annamycin can demonstrate superior efficacy to current standards of care, it could capture a significant market share.
Moleculin's multiple regulatory designations for Annamycin, including Fast Track Status and Orphan Drug Designation, provide additional development and marketing advantages that could accelerate its path to market and profitability if approved.
While the news is certainly positive for Moleculin, investors should remain cognizant of the inherent risks in late-stage oncology drug development, including the possibility of trial failure or regulatory setbacks.
Based on an encouraging discussion in the End of Phase 1B/2 Meeting with FDA the Company plans to:
Proceed with a pivotal, adaptive Phase 3 clinical trial (the "MIRACLE" trial) designed for possible accelerated approval of Annamycin in combination with cytarabine for the treatment of relapsed or refractory AML;
Run such future studies globally and in the US above the lifetime maximum allowable anthracycline dose; and
Provide the FDA with additional data supporting the selection of the optimal dosing level via the adaptive design in the MIRACLE trial
"We thank the FDA's Divisions of Hematologic Malignancies I and Cardiology and Nephrology, as well as related divisions, for a very constructive EOP1B/2 meeting and for their valuable feedback. Armed with this, we are now able to finalize plans for a pivotal approval pathway in AML," commented Walter Klemp, Chairman and Chief Executive Officer of Moleculin. "Importantly, consistent with the FDA's recommendations, the adaptive Phase 3 trial will rely solely on CR (complete remission) at day 30 as the primary endpoint versus placebo, a standard we are confident Annamycin will meet and that provides an opportunity for accelerated approval."
Mr. Klemp continued: "We now also have additional confidence that our planned pivotal trial should be able to generate data supportive of a true value inflection point for shareholders in a timely manner. We plan to utilize a double-blind, placebo-controlled design, where the control arm is high dose cytarabine (HiDAC) plus placebo. There is considerable historical data on the use of HiDAC. You can see in this graphic that, compared to this historical data, AnnAraC has already demonstrated more than double the CR rate. The MIRACLE trial will initially focus on 2nd line treatment for R/R AML subjects and then follow-up with treatment for 3rd line R/R AML."
"This approach should also allow us to use this trial for approval in
The Company obtained valuable input from the FDA and having resolved a number of key issues, believes that it has significantly de-risked the pathway to approval. The MIRACLE study, subject to appropriate future filings with and potential additional feedback from the FDA and their foreign equivalents, is expected to initially utilize an adaptive design whereby the first 75 patients will be randomized to receive HiDAC combined with either placebo, 190 mg/m2 of Annamycin, or 230 mg/m2 of Annamycin. At that point, the trial will be unblinded to select the Optimum Dose for Annamycin. For the second half of the trial, approximately 120 additional patients will be randomized to receive either HiDAC plus placebo or HiDAC plus the Optimum Dose of Annamycin. The selection of the Optimum Dose will be based not only on the absence of dose limiting toxicities but also on the overall balance of safety, pharmacokinetics and efficacy, consistent with the FDA's new Project Optimus initiative.
Mr. Klemp concluded: "The FDA also wants to see the durability of response (DoR) and overall survival (OS) as secondary endpoints, as well as data for patients beyond 2nd line, which is why our plan includes a follow-on MIRACLE2 trial in 3rd line patients starting once the optimum dose is established in the MIRACLE trial. From a Company perspective, we believe this approach is the best of all worlds. We are not only making the leap into being a Phase 3 company, but our planned approval is also based on a primary endpoint comparing to a control that we are optimistic we can beat with the ability to report unblinded progress after just 75 patients. We are truly excited to launch the MIRACLE trial."
Moleculin Planned Significant Milestones
The Company has established plans for the following milestones:
- 2H 2024 – Begin contracting with MIRACLE trial sites
- Q1 2025 – First subject treated in MIRACLE trial
- Mid 2026 – Interim data (n=75) unblinded and Optimum Dose set for MIRACLE trial
- 2026 – Begin enrollment of 3rd line subjects in MIRACLE2
- 2027 – Enrollment ends in 2nd line subjects
- 2028 – Final Data for 2nd line subjects in MIRACLE
- 2H 2028 – Begin submission of a new drug application (NDA) the treatment of R/R AML for accelerated approval on primary endpoint of CR from MIRACLE
Annamycin currently has Fast Track Status and Orphan Drug Designation from the US Food and Drug Administration for the treatment of relapsed or refractory acute myeloid leukemia, in addition to Orphan Drug Designation for the treatment of soft tissue sarcoma. Furthermore, Annamycin has Orphan Drug Designation for the treatment of relapsed or refractory acute myeloid leukemia from the European Medicines Agency (EMA). For more information about the ongoing MB-106 Phase 1B/2 trial, visit clinicaltrialsregister.eu and reference EudraCT 2020-005493-10 or clinicaltrials.gov and reference NCT05319587.
About Moleculin Biotech, Inc.
Moleculin Biotech, Inc. is a clinical stage pharmaceutical company with a growing pipeline, including Phase 2 clinical programs, for hard-to-treat tumors and viruses. The Company's lead program, Annamycin is a next-generation anthracycline designed to avoid multidrug resistance mechanisms and to eliminate the cardiotoxicity common with currently prescribed anthracyclines. Annamycin is currently in development for the treatment of acute myeloid leukemia (AML) and soft tissue sarcoma (STS) lung metastases. All interim and preliminary data related to its active clinical trials are subject to change until a clinical study report is published.
Additionally, the Company is developing WP1066, an Immune/Transcription Modulator capable of inhibiting p-STAT3 and other oncogenic transcription factors while also stimulating a natural immune response, targeting brain tumors, pancreatic and other cancers. Moleculin is also engaged in the development of a portfolio of antimetabolites, including WP1122 for the potential treatment of viruses, as well as certain cancer indications.
For more information about the Company, please visit www.moleculin.com and connect on X, LinkedIn and Facebook.
Forward-Looking Statements
Some of the statements in this release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, which involve risks and uncertainties. Although Moleculin believes that the expectations reflected in such forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward-looking statements. Moleculin has attempted to identify forward-looking statements by terminology including 'believes,' 'estimates,' 'anticipates,' 'expects,' 'plans,' 'projects,' 'intends,' 'potential,' 'may,' 'could,' 'might,' 'will,' 'should,' 'approximately' or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, including those discussed under Item 1A. "Risk Factors" in our most recently filed Form 10-K filed with the Securities and Exchange Commission (SEC) and updated from time to time in our Form 10-Q filings and in our other public filings with the SEC. Any forward-looking statements contained in this release speak only as of its date. We undertake no obligation to update any forward-looking statements contained in this release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events.
Investor Contact:
JTC Team, LLC
Jenene Thomas
(833) 475-8247
MBRX@jtcir.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/moleculin-announces-plans-for-miracle-phase-3-pivotal-trial-302211451.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/moleculin-announces-plans-for-miracle-phase-3-pivotal-trial-302211451.html
SOURCE Moleculin Biotech, Inc.
FAQ
What is the MIRACLE trial for Moleculin Biotech (MBRX)?
When does Moleculin (MBRX) expect to begin the MIRACLE Phase 3 trial?
What is the primary endpoint for Moleculin's (MBRX) MIRACLE Phase 3 trial?