Moleculin Announces Additional Positive Preliminary Interim Data from AML Clinical Trial
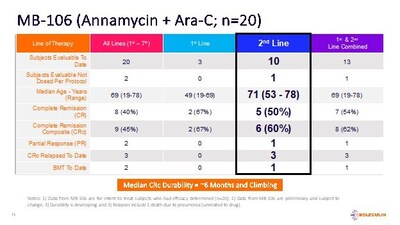
Moleculin Biotech reported additional positive interim data from its Phase 1B/2 clinical trial evaluating Annamycin in combination with Cytarabine (AnnAraC) for acute myeloid leukemia (AML). The trial enrolled 22 subjects, with 20 completing efficacy evaluations. The results showed a composite complete remission (CRc) in 45% of subjects, a median durability of response (mDOR) of 6 months and increasing, and no cardiotoxicity. In a 2nd line setting, AnnAraC achieved a median overall survival (mOS) of 6 months and 50% complete remissions (CR). The data was presented at the European Hematology Association (EHA) 2024 Hybrid Congress and showcased promising efficacy outcomes, especially for poor prognosis patients.
- 45% of subjects achieved a composite complete remission (CRc).
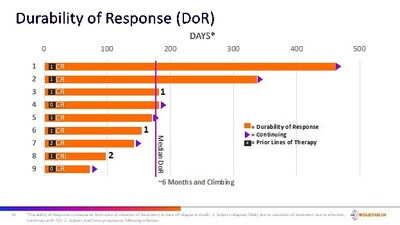
- Median durability of response (mDOR) is 6 months and increasing.
- 50% complete remissions (CR) and 60% CRc in 2nd line subjects.
- AnnAraC achieved a median overall survival (mOS) of 6 months and increasing.
- No cardiotoxicity observed.
- Promising preliminary data for poor prognosis patients.
- Annamycin has Fast Track Status and Orphan Drug Designation from the FDA and EMA.
- Data is still preliminary and subject to change.
- Two subjects discontinued early due to allergic reactions.
- The trial's efficacy outcomes are not yet statistically relevant.
Insights
The latest findings from Moleculin Biotech regarding Annamycin, particularly in its combination with Cytarabine (AnnAraC) for treating acute myeloid leukemia (AML), offer promising interim data. The response rates and survival statistics, especially among poor prognosis subgroups, are noteworthy. For context, composite complete remission (CRc) rates of 45% in evaluable subjects and 60% in second-line treatment subjects are significant. The lack of cardiotoxicity is a important advantage, given the severe cardiac side effects associated with many chemotherapeutic agents.
The median durability of response (mDOR) of approximately 6 months and increasing is especially promising. While preliminary and requiring further validation, this data can signal a breakthrough, particularly in the difficult-to-treat relapsed/refractory (R/R) AML population. These interim results deserve close attention as they could reshape treatment paradigms for AML. However, retail investors should await more mature data before making investment decisions.
From a financial perspective, Moleculin's Annamycin trial results could drive significant investor interest and affect the company's valuation. Interim data indicating 50% complete remission (CR) in second-line treatment patients and the absence of cardiotoxicity can enhance market confidence. These factors could lead to an increase in stock prices as they underline the potential for Annamycin to meet a critical unmet need in AML therapy.
Given the drug’s Fast Track and Orphan Drug Designation by the FDA, positive trial outcomes can expedite the regulatory approval process, potentially leading to faster commercialization and revenue generation. Investors should consider the long-term prospects, as the successful demonstration of Annamycin's efficacy and safety could result in substantial market share and profitability for Moleculin.
As an oncology expert, the interim results of Annamycin in combination with Cytarabine for AML are exciting. The presented data show that a significant proportion of subjects, including those with poor cytogenetic profiles, achieve meaningful clinical responses. Specifically, the composite complete remission (CRc) rate of 45% across evaluable subjects is impressive, given the challenging nature of the disease in these patients.
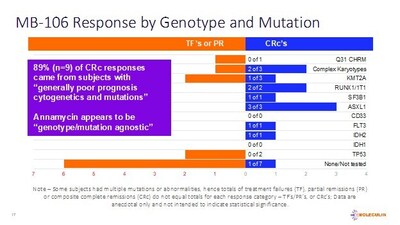
The fact that 89% of CRc subjects had poor prognosis cytogenetics emphasizes the potential of AnnAraC to alter the clinical course of AML. Moreover, the absence of cardiotoxicity is crucial, as it mitigates a significant risk associated with many existing chemotherapy regimens. This positions Annamycin as a potentially safer and more effective treatment option, warranting further clinical investigation and eventual integration into AML treatment protocols.
–
– Current median durability of response (mDOR) of CRc's = 6 months and increasing (n=9)
– In 2nd line subjects (n=10) Annamycin in combination with Cytarabine (AnnAraC) achieved an estimated median overall survival (mOS) of 6 months and increasing plus 5 CR's (
– Recruitment increased to 22 subjects and CRc's in all subjects evaluable for efficacy (n=20) was
– Annamycin continues to demonstrate no cardiotoxicity
– Data presented at European Hematology Association (EHA) 2024 Hybrid Congress and Company KOL Meeting in
To date, a total of 22 subjects have been enrolled (the Intent-to-Treat population, ITT), 20 (Lines 1st-7th) of whom have completed efficacy evaluations with 9 subjects (
Of the 10 ITT subjects for whom AnnAraC was administered in the 2nd line setting, 5 achieved a CR (
Additionally,
"We continue to be highly encouraged by the positive growing body of preliminary clinical data demonstrated by Annamycin in the treatment of patients with AML," commented Walter Klemp, Chairman and Chief Executive Officer of Moleculin. "While still preliminary, we believe the efficacy to date including the climbing durability of response demonstrated by AnnAraC in 2nd line patients continues to significantly exceed the performance reported by any drug currently approved for use in 2nd line AML. We are incredibly pleased with the progress of the trial and the data and continue to advance our preparations for an End of Phase 2 meeting with FDA."
"There remains a significant unmet need for safe and effective therapies for R/R AML. These data are exciting, and I believe further underscore the potential of Annamycin to provide patients and physicians with a promising treatment option in AML," concluded Mr. Klemp.
Currently, the median age of subjects in MB-106 is 69 years. Not including the two most recent subjects, a total of 17 subjects had relapsed/refractory AML and 3 subjects were first line treatment. Two subjects discontinued early due to allergic reactions. All subjects who completed treatment had undergone post-therapy bone marrow assessment (Day 15 or later). No clinically significant signs of cardiotoxicity were noted during or after treatment in any of the subjects enrolled. The combination was well tolerated with myelosuppression and infections being the main adverse events (AEs). All data from MB-106 is preliminary and subject to change.
Data presented at the European Hematology Association (EHA) 2024 Hybrid Congress:
As previously announced, the poster titled "LIPOSOMAL ANNAMYCIN (L-ANN) IN COMBINATION WITH CYTARABINE FOR TREATMENT OF PATIENTS WITH ACUTE MYELOID LEUKAEMIA (AML) REFRACTORY TO OR RELAPSED (R/R) AFTER INDUCTION THERAPY (MB-106 STUDY)," was presented as part of the "Acute myeloid leukemia – Clinical" session by Wolfram C. M. Dempke, MD, PhD, MBA, European Chief Medical Officer of Moleculin. The poster presentation mentioned above will be posted on the Company's website and filed on Form 8-K with the Securities and Exchange Commission.
Annamycin currently has Fast Track Status and Orphan Drug Designation from the
About Moleculin Biotech, Inc.
Moleculin Biotech, Inc. is a clinical stage pharmaceutical company with a growing pipeline, including Phase 2 clinical programs, for hard-to-treat tumors and viruses. The Company's lead program, Annamycin is a next-generation anthracycline designed to avoid multidrug resistance mechanisms and to eliminate the cardiotoxicity common with currently prescribed anthracyclines. Annamycin is currently in development for the treatment of acute myeloid leukemia (AML) and soft tissue sarcoma (STS) lung metastases. All interim and preliminary data related to its active clinical trials are subject to change.
Additionally, the Company is developing WP1066, an Immune/Transcription Modulator capable of inhibiting p-STAT3 and other oncogenic transcription factors while also stimulating a natural immune response, targeting brain tumors, pancreatic and other cancers. Moleculin is also engaged in the development of a portfolio of antimetabolites, including WP1122 for the potential treatment of viruses, as well as certain cancer indications.
For more information about the Company, please visit www.moleculin.com and connect on Twitter, LinkedIn and Facebook.
Forward-Looking Statements
Some of the statements in this release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, which involve risks and uncertainties. Although Moleculin believes that the expectations reflected in such forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward-looking statements. Moleculin has attempted to identify forward-looking statements by terminology including 'believes,' 'estimates,' 'anticipates,' 'expects,' 'plans,' 'projects,' 'intends,' 'potential,' 'may,' 'could,' 'might,' 'will,' 'should,' 'approximately' or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, including those discussed under Item 1A. "Risk Factors" in our most recently filed Form 10-K filed with the Securities and Exchange Commission (SEC) and updated from time to time in our Form 10-Q filings and in our other public filings with the SEC. Any forward-looking statements contained in this release speak only as of its date. We undertake no obligation to update any forward-looking statements contained in this release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events.
Investor Contact:
JTC Team, LLC
Jenene Thomas
(833) 475-8247
MBRX@jtcir.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/moleculin-announces-additional-positive-preliminary-interim-data-from-aml-clinical-trial-302172685.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/moleculin-announces-additional-positive-preliminary-interim-data-from-aml-clinical-trial-302172685.html
SOURCE Moleculin Biotech, Inc.
FAQ
What is the recent update on Moleculin's AML clinical trial?
What is the median overall survival for 2nd line subjects in Moleculin's trial?
Has Annamycin shown any cardiotoxicity in the trial?
How many subjects achieved a complete remission in Moleculin's AML trial?