Lipocine Announces Positive Oral Brexanolone Quantitative EEG Results
Lipocine Inc. (NASDAQ: LPCN) announced positive data from its quantitative Electroencephalogram (qEEG) study of oral brexanolone, a neuroactive steroid being developed for post-partum depression (PPD). The study, conducted on 12 healthy postmenopausal females, showed robust central nervous system (CNS) activity with concentration- and time-dependent post-dose changes in qEEG.
Key findings include:
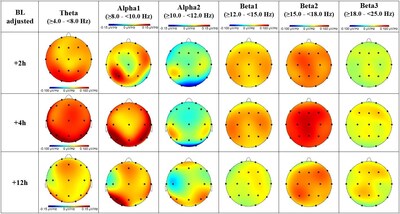
- Significant increases in theta and alpha1 band power in posterior cortical regions
- Decrease in alpha2 band power
- Considerable beta band amplitude increase across all cortical brain areas
- Rapid onset of effects (as early as 2 hours) with maximum impact at 4 hours post-dose
- Effects lasting up to 12 hours post-dose
These results confirm GABAA positive allosteric modulation and support the potential utility of oral brexanolone in treating various neuropsychiatric indications, including depression, anxiety, essential tremor, and epilepsy. The safety profile was consistent with previous clinical studies, showing minimal CNS depressant effects.
Lipocine Inc. (NASDAQ: LPCN) ha annunciato dati positivi dal suo studio quantitativo dell'Elettroencefalogramma (qEEG) sull'uso orale di brexanolone, uno steroide neuroattivo in fase di sviluppo per la depressione post-partum (PPD). Lo studio, condotto su 12 donne postmenopausali sane, ha mostrato una robusta attività del sistema nervoso centrale (CNS) con cambiamenti post-dose nel qEEG che dipendono dalla concentrazione e dal tempo.
I risultati chiave includono:
- Aumenti significativi della potenza delle bande theta e alpha1 nelle regioni corticali posteriori
- Riduzione della potenza della banda alpha2
- Aumento considerevole dell'ampiezza della banda beta in tutte le aree corticali del cervello
- Inizio rapido degli effetti (già dopo 2 ore) con impatto massimo a 4 ore dopo la dose
- Effetti che durano fino a 12 ore dopo la dose
Questi risultati confermano la modulazione alosterica positiva del GABAA e supportano il potenziale utilizzo del brexanolone orale nel trattamento di varie indicazioni neuropsichiatriche, tra cui depressione, ansia, tremore essenziale ed epilessia. Il profilo di sicurezza è risultato coerente con studi clinici precedenti, mostrando effetti depressivi minimi sul CNS.
Lipocine Inc. (NASDAQ: LPCN) anunció datos positivos de su estudio sobre Electroencefalograma Cuantitativo (qEEG) del brexanolona oral, un esteroide neuroactivo que se está desarrollando para la depresión postparto (PPD). El estudio, realizado en 12 mujeres posmenopáusicas sanas, mostró una robusta actividad en el sistema nervioso central (CNS) con cambios post-dosis en el qEEG que dependen de la concentración y del tiempo.
Los hallazgos clave incluyen:
- Aumentos significativos en la potencia de las bandas theta y alpha1 en las regiones corticales posteriores
- Disminución de la potencia de la banda alpha2
- Aumento considerable de la amplitud de la banda beta en todas las áreas corticales del cerebro
- Inicio rápido de los efectos (tan pronto como a las 2 horas) con impacto máximo a las 4 horas post-dosis
- Efectos que duran hasta 12 horas post-dosis
Estos resultados confirman la modulación alostérica positiva de GABAA y apoyan el potencial uso del brexanolona oral para tratar diversas indicaciones neuropsiquiátricas, incluyendo depresión, ansiedad, temblor esencial y epilepsia. El perfil de seguridad fue consistente con estudios clínicos anteriores, mostrando efectos depresores mínimos en el CNS.
리포신 Inc. (NASDAQ: LPCN)은 출산 후 우울증 (PPD)을 치료하기 위해 개발 중인 신경 활성 스테로이드인 구강용 브렉사놀론에 대한 정량적 뇌파검사(qEEG) 연구의 긍정적인 데이터를 발표했습니다. 12명의 건강한 폐경 후 여성들을 대상으로 실시된 이 연구는 중추신경계(CNS)의 강력한 활성화와 용량 및 시간에 따라 변화하는 qEEG의 복용 후 변화를 보여주었습니다.
주요 발견 사항은 다음과 같습니다:
- 후측 피질 영역에서 세타 및 알파1 대역 전력의 유의미한 증가
- 알파2 대역 전력의 감소
- 모든 피질 뇌 영역에서 베타 대역 진폭의 상당한 증가
- 2시간 이내에 빠른 효과 발현, 최대 효과는 복용 후 4시간에 나타남
- 복용 후 최대 12시간까지 지속되는 효과
이 결과들은 GABAA 양성 알로스테리 조절을 확인하고, 다양한 신경정신과적 적응증, 특히 우울증, 불안, 본태성 떨림 및 간질 치료를 위한 경구용 브렉사놀론의 잠재적 유용성을 지원합니다. 안전성 프로필은 이전 임상 연구와 일관되며, 최소한의 CNS 억제 효과를 나타냅니다.
Lipocine Inc. (NASDAQ: LPCN) a annoncé des données positives de son étude sur l'électroencéphalogramme quantitatif (qEEG) concernant le brexanolone oral, un stéroïde neuroactif en développement pour la dépression post-partum (PPD). L'étude, menée auprès de 12 femmes ménopausées en bonne santé, a montré une activité robuste du système nerveux central (CNS) avec des changements post-dose dans le qEEG qui dépendent de la concentration et du temps.
Les résultats clés comprennent :
- Augmentations significatives de la puissance des bandes theta et alpha1 dans les régions corticales postérieures
- Diminution de la puissance de la bande alpha2
- Augmentation considérable de l'amplitude de la bande beta dans toutes les zones corticales du cerveau
- Effets d'apparition rapide (dès 2 heures) avec un impact maximal à 4 heures après la dose
- Effets durables jusqu'à 12 heures après la dose
Ces résultats confirment la modulation allostérique positive de GABAA et soutiennent l'utilité potentielle du brexanolone oral dans le traitement de diverses indications neuropsychiatriques, y compris la dépression, l'anxiété, le tremblement essentiel et l'épilepsie. Le profil de sécurité était cohérent avec les études cliniques précédentes, montrant des effets dépresseurs minimes sur le CNS.
Lipocine Inc. (NASDAQ: LPCN) hat positive Daten aus seiner quantitativen Elektroenzephalogramm (qEEG) Studie zu oralem Brexanolon veröffentlicht, einem neuroaktiven Steroid, das zur Behandlung von postpartaler Depression (PPD) entwickelt wird. Die Studie, die an 12 gesunden postmenopausalen Frauen durchgeführt wurde, zeigte eine robuste Aktivität des zentralen Nervensystems (CNS) mit konzentrations- und zeitabhängigen post-dosischen Veränderungen im qEEG.
Wichtige Ergebnisse umfassen:
- Signifikante Zunahmen der Theta- und Alpha1-Bandkraft in den posterioren kortikalen Regionen
- Abnahme der Alpha2-Bandkraft
- Deutlicher Anstieg der Beta-Bandamplitude in allen kortikalen Hirnarealen
- Schneller Wirkungseintritt (bereits nach 2 Stunden) mit maximaler Wirkung 4 Stunden nach der Dosis
- Wirkung, die bis zu 12 Stunden nach der Dosis anhält
Diese Ergebnisse bestätigen die positive allosterische Modulation von GABAA und unterstützen die potenzielle Anwendung von oralem Brexanolon zur Behandlung verschiedener neuropsychiatrischer Indikationen, einschließlich Depression, Angst, essentieller Tremor und Epilepsie. Das Sicherheitsprofil war konsistent mit vorherigen klinischen Studien und zeigte minimale dämpfende Effekte auf das CNS.
- Positive qEEG results confirming target engagement of oral bioidentical brexanolone
- Potential utility in treating multiple psychiatry and neurology indications
- Rapid onset of effects (as early as 2 hours) with lasting impact up to 12 hours post-dose
- Favorable safety profile with minimal CNS depressant effects
- None.
Insights
The qEEG study results for oral brexanolone are highly promising, demonstrating robust CNS activity and GABAA modulation. Key findings include:
- Significant increases in theta and alpha1 band power in posterior cortical regions
- Decreased alpha2 band power
- Widespread increase in beta band amplitude, particularly beta2
- Rapid onset (2 hours) with peak effects at 4 hours and lasting up to 12 hours
These EEG changes are consistent with effective treatments for depression, anxiety, tremor and seizures. The favorable safety profile and minimal CNS depressant effects further support its potential. For investors, this data significantly de-risks Lipocine's oral brexanolone program, potentially opening up multiple high-value CNS indications beyond postpartum depression. The biomarker confirmation of target engagement increases the probability of success in future clinical trials, which could be a major value driver for LPCN stock.
This positive qEEG data represents a significant milestone for Lipocine, potentially expanding its market opportunity beyond postpartum depression. Key financial implications include:
- Increased likelihood of clinical success, reducing development risk
- Potential for multiple high-value CNS indications, expanding the addressable market
- Competitive advantage in oral delivery of brexanolone vs. existing IV formulations
- Possible increased interest from potential partners or acquirers
With a market cap of only
- Quantitative Electroencephalogram (qEEG) in healthy subjects administered single doses of oral brexanolone, a neuroactive steroid (NAS), confirmed GABAA modulation
- Rapid and durable CNS target engagement confirms effective oral delivery of bioidentical brexanolone
- Promising results support continued development of oral brexanolone for the treatment of neuropsychiatric disorders
"We are pleased with the qEEG results that confirm target engagement of oral bioidentical brexanolone, which suggests potential utility in treating numerous psychiatry indications, including depression and anxiety, and neurology indications such as essential tremor and epilepsy," said Dr. Mahesh Patel, President and CEO of Lipocine Inc. "These positive biomarker results and favorable safety profile support further development of oral brexanolone."
This Phase 1 study evaluated qEEG spectral power changes after administration of oral brexanolone. Healthy postmenopausal females (N=12) were administered single doses of oral brexanolone. EEG recordings and blood samples were collected pre- and post-dose (2, 4 and 12 hours). EEG recordings were obtained using a wireless, 19-electrode EEG monitoring device (Zeto Inc.,
Following a single clinically relevant dose of oral brexanolone, subjects showed mean changes in all oscillatory spectral power bands. As shown in Fig. 1, theta and alpha1 band power were significantly increased in posterior cortical regions, while alpha2 band power decreased. There was considerable beta band amplitude increase, including significant increase in beta2 amplitude across all cortical brain areas Most of the treatment-related EEG changes were rapid occurring as early as 2 hours, with maximum and significant mean contrast values at 4 hours post dose (consistent with Tmax) with appreciable effects lasting 12 hours post-dose.
The observed qEEG changes following oral brexanolone administration are consistent with therapies effective in managing depression, anxiety, tremor, and seizures.1-5
Oral brexanolone was well-tolerated in this Phase 1 study. The safety profile was consistent with safety data from clinical studies previously conducted by Lipocine with minimal CNS depressant effects.
Lipocine plans to present the additional details and analyses from this EEG study at upcoming scientific meetings.
About Quantitative Electroencephalogram (qEEG)
Quantitative Electroencephalogram (qEEG) is an advanced neuroimaging technique used to measure electrical activity in the brain with a high degree of precision and detail. qEEG uses mathematical and statistical methods to analyze the electrical signals generated by the brain and convert them into quantitative metrics. By translating these signals into a digital format, qEEG allows for the identification and assessment of specific brain wave frequencies -- such as delta, theta, alpha, beta, and gamma waves -- associated with different states of cognition, emotion, and behavior. This analysis allows researchers to detect subtle changes in brain function that may be induced by a drug, providing important insights into its mechanism of action. In the context of drug development, qEEG is used to evaluate the effect of new therapies on the central nervous system (CNS) by monitoring shifts in brain wave patterns that correlate with therapeutic outcomes. This helps determine whether the drug is acting on the desired neural circuits, provides early evidence of efficacy, and may support dose selection for future clinical trials.
About Lipocine
Lipocine is a biopharmaceutical company leveraging its proprietary technology platform to augment therapeutics through effective oral delivery to develop differentiated products. Lipocine has drug candidates in development as well as drug candidates for which we are exploring partnerships. Our drug candidates represent enablement of differentiated, patient friendly oral delivery options for favorable benefit to risk profile which target large addressable markets with significant unmet medical needs.
Lipocine's clinical development candidates include: LPCN 1154, oral brexanolone, for the potential treatment of postpartum depression, LPCN 2101 for the potential treatment of epilepsy, LPCN 2203 an oral candidate targeted for the management of essential tremor, LPCN 2401 an oral proprietary anabolic androgen receptor agonist, as an adjunct therapy to incretin mimetics, as an aid for improved body composition in obesity management and LPCN 1148, a novel androgen receptor agonist prodrug for oral administration targeted for the management of symptoms associated with liver cirrhosis. Lipocine is exploring partnering opportunities for LPCN 1107, our candidate for prevention of preterm birth, LPCN 1154, for rapid relief of postpartum depression, LPCN 2401 for obesity management, LPCN 1148, for the management of decompensated cirrhosis, and LPCN 1144, our candidate for treatment of non-cirrhotic NASH. TLANDO, a novel oral prodrug of testosterone containing testosterone undecanoate developed by Lipocine, is approved by the FDA for conditions associated with a deficiency of endogenous testosterone, also known as hypogonadism, in adult males. For more information, please visit www.lipocine.com.
Forward-Looking Statements
This release contains "forward-looking statements" that are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 and include statements that are not historical facts regarding our product development efforts, our strategic plans for developing products, our ability to monetize product candidates, including through entering into partnering arrangements, our product candidates and related clinical trials, the achievement of milestones within and completion of clinical trials, the timing and completion of regulatory reviews, outcomes of clinical trials of our product candidates, and the potential uses and benefits of our product candidates. Investors are cautioned that all such forward-looking statements involve risks and uncertainties, including, without limitation, the risks that we may not be successful in developing product candidates, we may not have sufficient capital to complete the development processes for our product candidates, we may not be able to enter into partnerships or other strategic relationships to monetize our non-core assets, the FDA will not approve any of our products, risks related to our products, expected product benefits not being realized, clinical and regulatory expectations and plans not being realized, new regulatory developments and requirements, risks related to the FDA approval process including the receipt of regulatory approvals, and our ability to utilize a streamlined approval pathway for LPCN 1154, the results and timing of clinical trials, patient acceptance of Lipocine's products, the manufacturing and commercialization of Lipocine's products, and other risks detailed in Lipocine's filings with the SEC, including, without limitation, its Form 10-K and other reports on Forms 8-K and 10-Q, all of which can be obtained on the SEC website at www.sec.gov. Lipocine assumes no obligation to update or revise publicly any forward-looking statements contained in this release, except as required by law.
- Meltzer-Brody et al. Lancet 2018; 392(10152): 1058-1070.
- Buchsbaum et al. Biol Psychiatry 1985; 20(8): 832-842.
- Ibanez et al. Plos One 2014; 9(3): e93159.
- Huang and Shen Clin Electroencephalography 1994; 24(4): 179-187
- Biondi et al. Sci Rep 2022; 12(1): 1919.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/lipocine-announces-positive-oral-brexanolone-quantitative-eeg-results-302272247.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/lipocine-announces-positive-oral-brexanolone-quantitative-eeg-results-302272247.html
SOURCE Lipocine Inc.