Lexaria's Patented Technology Improved the Oral Performance of the Rybelsus(R)-Branded GLP-1 drug Semaglutide
- DehydraTECH GLP-1 blood semaglutide levels were ~261% higher than that of the Control at 20 minutes post-administration
- DehydraTECH GLP-1 peak levels of semaglutide in blood were 43% higher than in Rybelsus
- DehydraTECH GLP-1 blood semaglutide levels were approximately 44% higher than the Control levels 24 hours post-dosing
- DehydraTECH GLP-1 processed semaglutide was better tolerated than Rybelsus tablets with reduced instances of moderate nausea and moderate diarrhea
- DehydraTECH GLP-1 was more effective at maintaining consistently reduced blood glucose levels even after eating a standardized meal and snack
- None.
Insights
The results indicating that DehydraTECH-powered semaglutide achieves sustained higher levels of the drug in the bloodstream and better blood glucose control compared to Rybelsus are significant. These findings suggest a potential for improved therapeutic outcomes for patients with diabetes. The reduction in side effects is particularly noteworthy, as gastrointestinal issues are common with GLP-1 therapies and can lead to treatment discontinuation.
From a medical research perspective, the enhanced bioavailability and efficacy demonstrated in this study could lead to a reevaluation of current GLP-1 treatment protocols. It's essential to consider the sample size and the need for further trials to validate these results across a broader population. However, the data suggests that DehydraTECH technology could be a game-changer in oral drug delivery, potentially improving patient compliance and outcomes.
Lexaria Bioscience Corp.'s announcement may have positive implications for its market position and stock valuation. The pharmaceutical industry places a premium on drug delivery technologies that can enhance the efficacy and patient experience of existing medications. Improved formulations of drugs like semaglutide can extend product lifecycles and create competitive advantages.
The market for diabetes treatment is substantial, with a global valuation in the billions and growing due to rising diabetes prevalence. A superior delivery method for semaglutide could capture a significant market share. Investors should monitor potential partnerships, licensing deals, or acquisitions that may arise from these findings. However, the long-term impact will depend on regulatory approvals and market adoption.
The reported improvements in drug delivery and glucose control are promising, but clinical trial design and regulatory considerations are critical. The study's robustness, including its design, methodology and statistical significance, will be scrutinized in the process of FDA review and potential market approval. It's crucial to assess whether the study aligns with FDA guidelines for bioequivalence and efficacy studies.
Future clinical trials will need to replicate these results in larger populations and over longer periods to satisfy regulatory requirements. The potential for expedited pathways or breakthrough designations could be considered if the technology demonstrates significant advantages over existing therapies. The long-term impact on Lexaria's business will be closely tied to these regulatory outcomes.
Final results from a human pilot study show DehydraTECHTM-powered semaglutide outperforms Rybelsus®:
- Sustained higher levels of semaglutide in blood;
- Better blood glucose control;
- Faster achievement of peak drug delivery; and
- Reduced side effects.
KELONA, BC / ACCESSWIRE / January 4, 2024 / Lexaria Bioscience Corp. (NASDAQ:LEXX & LEXXW) (the "Company" or "Lexaria"), a global innovator in drug delivery platforms announces positive final results from its recently completed human Pilot Study #1 (the "Study") evaluating DehydraTECHTM technology for the oral delivery of the glucagon-like peptide-1 ("GLP-1") drug semaglutide available commercially in the branded product Rybelsus®.
The Study was performed by a prominent university research center comparing a single 7 mg semaglutide dose of a Rybelsus® tablet ("Control") to a matching dose from Rybelsus that had been compound formulated in capsule form using DehydraTECH processing technology enhancements ("DehydraTECH GLP-1").
In general, the DehydraTECH processing enabled improvements in delivery of semaglutide to the bloodstream, and the improvements in controlling blood sugar were more pronounced in the final combined results of the Study than they were in the first half of the Study as reported in press releases issued November 27 and November 28, 2023.
Blood Levels of Semaglutide
The first post-baseline blood was sampled 20 minutes after oral administration and, at that point in time, the DehydraTECH GLP-1 blood semaglutide level was ~
Blood Semaglutide Levels (mmol/L)
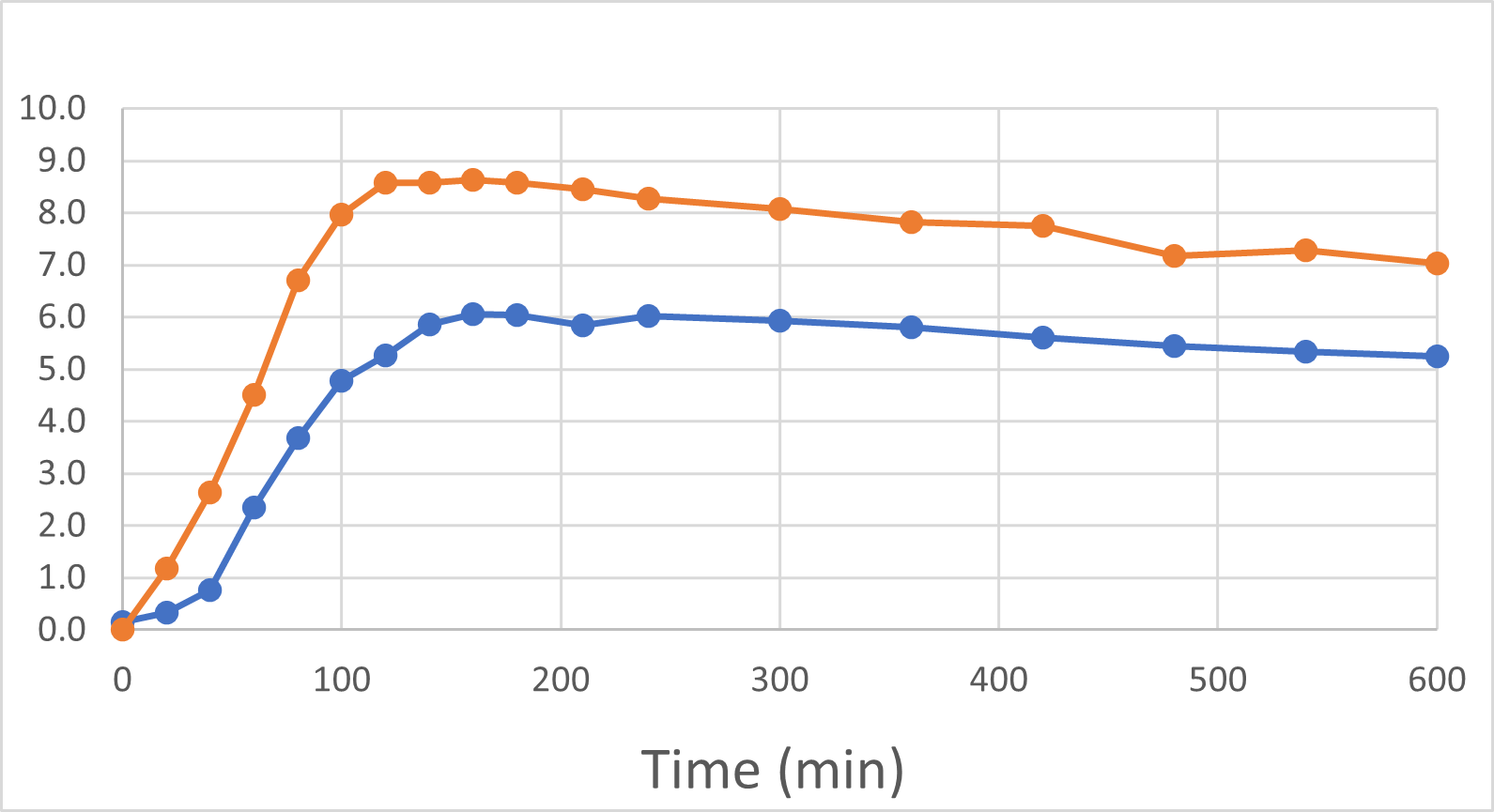
Rybelsus Control (blue) 7mg (n=7) DehydraTECH (orange) GLP-1 7mg (n=7)
24 hours after the ingestion of the single dose, the DehydraTECH GLP-1 blood semaglutide levels were approximately
Side Effects
The DehydraTECH GLP-1 processed semaglutide was generally better tolerated than the Rybelsus® tablets themselves. Only the Rybelsus® tablets resulted in instances of moderate nausea and moderate diarrhea, whereas no such instances were reported upon DehydraTECH-semaglutide dosing.
Blood Glucose Levels
It is accepted by the Food and Drug Administration ("FDA") that, "one role of GLP-1 is to prompt the body to produce more insulin, which reduces blood glucose (sugar)." Because blood glucose levels are a key consideration in control of diabetes and other health conditions, the Study measured blood glucose levels at each of the 19 sample time points.
Blood Glucose Levels (mmol/L)
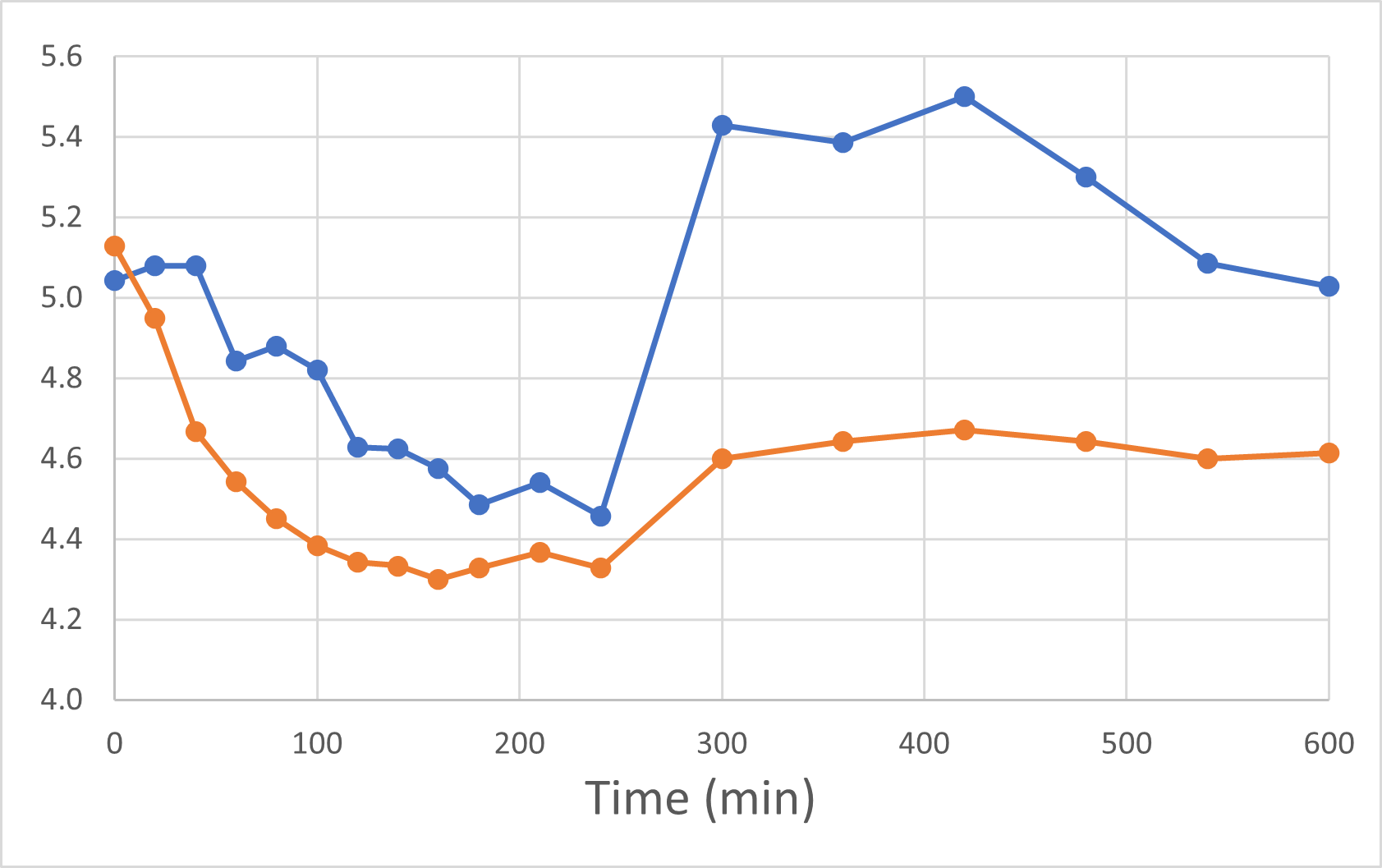
Rybelsus Control (blue) 7mg (n=7) DehydraTECH (orange) GLP-1 7mg (n=7)
The Control group evidenced inconsistent blood glucose reduction that did not prevent blood glucose spikes after eating. DehydraTECH GLP-1 reduced blood glucose to lower levels and was much more effective at maintaining consistently reduced blood glucose levels even after eating a standardized meal at the 240-minute mark and a standardized snack at the 360-minute mark. Actual data levels for each hour after Rybelsus® (control) and DehydraTECH drug administration are shown below.
| Blood Glucose Levels (mmol/L) | ||||
Minutes | Control Mean | Change from Time Zero | DehydraTECH Mean | Change from Time Zero |
0 | 5.04 | 5.13 | ||
60 | 4.84 | - | 4.54 | - |
120 | 4.63 | - | 4.34 | - |
180 | 4.49 | - | 4.33 | - |
240 | 4.46 | - | 4.33 | - |
300 | 5.43 | + | 4.60 | - |
360 | 5.39 | + | 4.64 | - |
420 | 5.5 | + | 4.67 | - |
480 | 5.3 | + | 4.64 | - |
540 | 5.09 | + | 4.60 | - |
600 | 5.03 | - | 4.61 | - |
24-Hour | 5.04 | 4.87 | - | |
Notably, even as long as 24 hours after dose administration, the blood glucose levels were reduced in the DehydraTECH GLP-1 group by
This Study is only meant to provide early-stage indicative information to Lexaria about the possibility of enhancing the pharmacokinetic and pharmacodynamic performance of orally delivered GLP-1 drugs to assist in guiding the Lexaria team in additional investigations. There was minor variability in the diets of the subjects at the 240-minute meal and 360-minute snack intervals noted above during the concentrated 10-hour post dosing monitoring period which could account for some of the differences in the test data, although meal and snack selection allowance was from a set of standardized choices.
Future Work
Due to the success of the Study, Lexaria is already preparing for other human and animal studies to continue the evaluation of DehydraTECH's effectiveness with GLP-1 drugs. This work will be conducted on an expedited basis given the urgent need for effective oral delivery of GLP-1 drugs and DehydraTECH's apparent ability to improve their performance. Lexaria will provide more details about its GLP-1 strategy in separate news.
About the Study
The Study was performed to provide an early-stage indication of whether DehydraTECH processing could improve oral drug delivery characteristics of the GLP-1 drug semaglutide sold as Rybelsus®. A single semaglutide dose of 7 mg of the Control was compared to the matching dose of the DehydraTECH GLP-1, swallowed by each subject after an overnight fasting period together with a 50 mL glass of water. The DehydraTECH GLP-1 formulation used in this Study was compound formulated strictly for research purposes. Seven healthy subjects were dosed with each test article following a cross over study design across two study visits.
About Semaglutide
Rybelsus® (semaglutide) is the only GLP-1 drug approved by the FDA for oral dosing to treat diabetes and weight loss. The FDA has also approved semaglutide marketed as Ozempic® and Wegovy®, administered by injection, to treat diabetes and weight loss. All three of these drugs are owned and manufactured by Novo Nordisk®.
About Lexaria Bioscience Corp.
Lexaria Bioscience Corp.'s patented drug delivery technology, DehydraTECH™, improves the way active pharmaceutical ingredients (APIs) enter the bloodstream through oral delivery. Since 2016, DehydraTECH has repeatedly demonstrated the ability to increase bio-absorption with cannabinoids, antiviral drugs, PDE5 inhibitors and more. DehydraTECH has also evidenced an ability to deliver some drugs more effectively across the blood brain barrier. Lexaria operates a licensed in-house research laboratory and holds a robust intellectual property portfolio with 38 patents granted and many patents pending worldwide. For more information, please visit www.lexariabioscience.com.
CAUTION REGARDING FORWARD-LOOKING STATEMENTS
This press release includes forward-looking statements. Statements as such term is defined under applicable securities laws. These statements may be identified by words such as "anticipate," "if," "believe," "plan," "estimate," "expect," "intend," "may," "could," "should," "will," and other similar expressions. Such forward-looking statements in this press release include, but are not limited to, statements by the Company relating to the Company's ability to carry out research initiatives, receive regulatory approvals or grants or experience positive effects or results from any research or study. Such forward-looking statements are estimates reflecting the Company's best judgment based upon current information and involve a number of risks and uncertainties, and there can be no assurance that the Company will actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements. As such, you should not place undue reliance on these forward-looking statements. Factors which could cause actual results to differ materially from those estimated by the Company include, but are not limited to, government regulation and regulatory approvals, managing and maintaining growth, the effect of adverse publicity, litigation, competition, scientific discovery, the patent application and approval process, potential adverse effects arising from the testing or use of products utilizing the DehydraTECH technology, the Company's ability to maintain existing collaborations and realize the benefits thereof, delays or cancellations of planned R&D that could occur related to pandemics or for other reasons, and other factors which may be identified from time to time in the Company's public announcements and periodic filings with the US Securities and Exchange Commission on EDGAR. The Company provides links to third-party websites only as a courtesy to readers and disclaims any responsibility for the thoroughness, accuracy or timeliness of information at third-party websites. There is no assurance that any of Lexaria's postulated uses, benefits, or advantages for the patented and patent-pending technology will in fact be realized in any manner or in any part. No statement herein has been evaluated by the Food and Drug Administration (FDA). Lexaria-associated products are not intended to diagnose, treat, cure or prevent any disease. Any forward-looking statements contained in this release speak only as of the date hereof, and the Company expressly disclaims any obligation to update any forward-looking statements or links to third-party websites contained herein, whether as a result of any new information, future events, changed circumstances or otherwise, except as otherwise required by law.
INVESTOR CONTACT:
George Jurcic - Head of Investor Relations
ir@lexariabioscience.com
Phone: 250-765-6424, ext 202
SOURCE: Lexaria Bioscience Corp.
View the original press release on accesswire.com
FAQ
What is the company name and ticker symbol mentioned in the press release about the human Pilot Study #1 evaluating DehydraTECH technology for the oral delivery of the GLP-1 drug semaglutide?
What were the key findings of the human Pilot Study #1 comparing DehydraTECH GLP-1 to Rybelsus?
How did the DehydraTECH GLP-1 blood semaglutide levels compare to the Control group at 20 minutes post-administration?
How did the DehydraTECH GLP-1 blood semaglutide levels compare to the Control group 24 hours post-dosing?
Were there any side effects reported in the study comparing DehydraTECH GLP-1 to Rybelsus?







