Lexaria Granted Important New Oral Nicotine Patent
Lexaria Bioscience Corp. (NASDAQ:LEXX, LEXXW) announced the granting of its 24th patent, titled "Compositions Infused with Nicotine Compounds and Methods of Use Thereof," enhancing its global intellectual property portfolio. This Australian patent allows the use of DehydraTECH technology for various nicotine forms, potentially increasing product value. Previous studies show DehydraTECH delivers nicotine significantly faster than conventional methods. Additionally, the company issued 36,700 stock options to staff at an exercise price of $3.39.
- Granting of 24th patent expands intellectual property, potentially increasing product value.
- DehydraTECH technology shows 10x to 20x faster nicotine delivery vs. controls.
- None.
Newest granted patent expands worldwide patent portfolio to 24
KELOWNA, BC / ACCESSWIRE / March 8, 2022 / Lexaria Bioscience Corp. (NASDAQ:LEXX)(NASDAQ:LEXXW) (the "Company" or "Lexaria"), a global innovator in drug delivery platforms announces it has been granted a new patent entitled "Compositions Infused with Nicotine Compounds and Methods of Use Thereof".
The new Australian patent expands upon Lexaria's international intellectual property rights to apply DehydraTECH enhancement technology to most oral forms of nicotine, including pills, tablets, lozenges, capsules, pouches, gums and sprays. The patent covers many different forms of nicotine including free base nicotine, nicotine salts, polymer resins of nicotine and other forms of nicotine complexes.
Given the rapid progress Lexaria is making towards development of world-leading oral nicotine products, the intellectual property protection afforded by this patent and other similar patent applications, could be meaningful towards building value.
In news announced on October 5, 2021, Lexaria demonstrated in its animal study NIC-A21-1 that nicotine oral pouches using DehydraTECH technology were 10x to 20x faster in reaching peak delivery of nicotine to bloodstream than controls. Findings using a DehydraTECH nicotine benzoate formulation relative to a concentration-matched control from that study are shown in the figure below.
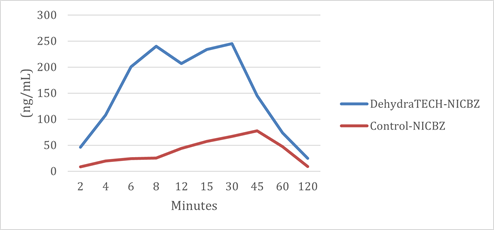
This is Lexaria's 24th overall granted patent receipt and is noteworthy for a number of reasons including it is the first patent awarded from Lexaria's 8th patent family. At times in Lexaria's history, the granting of a first patent in a new patent family has led to additional subsequent patents from within that same family. Corresponding patent applications are pending within this patent family elsewhere in the world including in the United States. A total of 9 claims have been awarded within this patent granted by the government of Australia that has an expiration date of April 9, 2039.
In other news, Lexaria announces that it has issued an aggregate 36,700 stock options to a total of six employees, consultants and independent directors bearing an exercise price of US
About Lexaria Bioscience Corp.
Lexaria Bioscience Corp.'s patented drug delivery technology, DehydraTECH™, improves the way active pharmaceutical ingredients (APIs) enter the bloodstream by promoting more effective oral delivery. Since 2016, DehydraTECH has repeatedly demonstrated the ability to increase bio-absorption with cannabinoids and nicotine by 5-10x and, in some instances with cannabinoids by as much as 27x compared to standard industry formulations, reduce time of onset from 1 - 2 hours to minutes, and mask unwanted tastes; and is also being evaluated for orally administered antiviral drugs, non-steroidal anti-inflammatory drugs (NSAIDs), PDE5 inhibitors and more. DehydraTECH has also evidenced an ability to deliver some drugs more effectively across the blood brain barrier. Lexaria operates a licensed in-house research laboratory and holds a robust intellectual property portfolio with 24 patents granted and over 50 patents pending worldwide. For more information, please visit www.lexariabioscience.com.
CAUTION REGARDING FORWARD-LOOKING STATEMENTS
This press release includes forward-looking statements. Statements as such term is defined under applicable securities laws. These statements may be identified by words such as "anticipate," "if," "believe," "plan," "estimate," "expect," "intend," "may," "could," "should," "will," and other similar expressions. Such forward-looking statements in this press release include, but are not limited to, statements by the company relating the Company's ability to carry out research initiatives, receive regulatory approvals or grants or experience positive effects or results from any research or study. Such forward-looking statements are estimates reflecting the Company's best judgment based upon current information and involve a number of risks and uncertainties, and there can be no assurance that the Company will actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements. As such, you should not place undue reliance on these forward-looking statements. Factors which could cause actual results to differ materially from those estimated by the Company include, but are not limited to, government regulation and regulatory approvals, managing and maintaining growth, the effect of adverse publicity, litigation, competition, scientific discovery, the patent application and approval process, potential adverse effects arising from the testing or use of products utilizing the DehydraTECH technology, the Company's ability to maintain existing collaborations and realize the benefits thereof, delays or cancellations of planned R&D that could occur related to pandemics or for other reasons, and other factors which may be identified from time to time in the Company's public announcements and periodic filings with the US Securities and Exchange Commission on EDGAR. There is no assurance that any of Lexaria's postulated uses, benefits, or advantages for the patented and patent-pending technology will in fact be realized in any manner or in any part. No statement herein has been evaluated by the Food and Drug Administration (FDA). Lexaria-associated products are not intended to diagnose, treat, cure or prevent any disease. Any forward-looking statements contained in this release speak only as of the date hereof, and the Company expressly disclaims any obligation to update any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise, except as otherwise required by law.
INVESTOR CONTACT:
George Jurcic - Head of Investor Relations
ir@lexariabioscience.com
Phone: 250-765-6424, ext 202
SOURCE: Lexaria Bioscience Corp.
View source version on accesswire.com:
https://www.accesswire.com/691968/Lexaria-Granted-Important-New-Oral-Nicotine-Patent
FAQ
What is Lexaria Bioscience's new patent about?
How does DehydraTECH technology improve nicotine delivery?
What is the significance of Lexaria's 24th patent?
What are the details of the stock options issued by Lexaria?







