INmune Bio Announces Plan to Submit FDA Biologics License Application (BLA) Seeking Approval of CORDStrom for Treatment of Recessive Dystrophic Epidermolysis Bullosa (RDEB)
Rhea-AI Summary
INmune Bio (NASDAQ: INMB) announces plans to submit a Biologics License Application (BLA) to the FDA for CORDStrom, targeting the treatment of Recessive Dystrophic Epidermolysis Bullosa (RDEB) in pediatric patients. The decision follows positive results from the MissionEB clinical trial, a double-blind, placebo-controlled study involving 30 pediatric patients.
CORDStrom demonstrated significant efficacy, particularly in reducing itch severity by over 27% at 6-months in severe cases, with improvements in skin integrity for younger patients. The treatment received both Rare Pediatric Disease Designation and Orphan Drug Designation from the FDA. The company estimates approximately 4,500 children with intermediate or severe RDEB in the US, UK, and EU could benefit from this therapy.
The company plans to submit the BLA in 2025, followed by Marketing Authorization Applications in the EU and UK in 2026. A 12-month open-label study is planned to continue supporting patients from the MissionEB trial.
Positive
- Successful completion of double-blind, placebo-controlled study with positive efficacy results
- 27% sustained reduction in itch severity at 6-months for severe cases
- Received both Rare Pediatric Disease and Orphan Drug Designations
- No serious adverse events reported during the trial
- Potential market of 4,500 children with intermediate or severe RDEB
- Eligible for Priority Review Voucher if approved by September 30, 2026
- 7 years of market exclusivity post-approval through Orphan Drug Designation
Negative
- BLA submission timeline extends into 2025
- EU/UK marketing applications delayed until 2026
- patient population size may restrict revenue potential
News Market Reaction
On the day this news was published, INMB declined 4.95%, reflecting a moderate negative market reaction.
Data tracked by StockTitan Argus on the day of publication.
- The Company reports results of a double-blinded, randomized, placebo-controlled study, known as “MissionEB,” investigating CORDStrom for treatment of RDEB in pediatric patients, which evidence a favorable benefit-risk profile in support of the intended applications for marketing authorization.
- CORDStrom, developed by INmune Bio is a patent pending, off the shelf, advanced mesenchymal stromal cell (MSC) platform developed to treat complex inflammatory diseases that has significant clinical development advantages over current MSC products.
- FDA grants CORDStrom a Rare Pediatric Disease Designation (RPDD) and Orphan Drug Designation (ODD) for treatment of epidermolysis bullosa (EB).
- Parallel efforts will seek marketing authorizations in the European Medicines Agency (EMA) and the Medicines and Healthcare products Regulatory Agency (MHRA).
- Investor Webcast Today at 8:30 a.m. ET.
Boca Raton, Florida, Feb. 10, 2025 (GLOBE NEWSWIRE) -- INmune Bio, Inc. (NASDAQ: INMB) (the “Company”), a clinical-stage inflammation and immunology company focused on developing treatments that harness the patient’s innate immune system to fight disease, announced today, following a Type C meeting with the U.S. Food and Drug Administration (FDA), its intent to submit a BLA in the US and Marketing Authorization Application (MAA) in the UK and EU supported by data from the MissionEB clinical trial investigating CORDStrom as a disease-modifying therapy for treating RDEB in pediatric patients.
RDEB is a rare, severely debilitating genetic disease, which has its onset in early childhood. Patients' skin is extremely fragile and is easily damaged, resulting in painful and itchy blistering wounds and scarring that can lead to aggressive and life-threatening skin cancer in adulthood. Long-term morbidity is driven by a debilitating itch and pain that significantly exacerbates wounds and deeply affects quality of life. The currently available treatments target active lesions via topical administration and have a limited benefit. The Company estimates roughly 4,500 children with intermediate or severe RDEB in the US, UK and EU may benefit from systemic CORDStrom therapy (all RDEB incidence: 95 per million live births, at least ~
The MissionEB study, led by Dr. Anna Martinez and team at the Great Ormond Street Hospital (GOSH) in collaboration with clinicians from Birmingham’s Children’s Hospital, was a double blind, placebo-controlled, cross-over study evaluating the safety and efficacy of CORDStrom in 30 pediatric patients (age <16 years) in the UK with intermediate or severe RDEB. Subjects were randomized to CORDStrom or placebo and received two intravenous infusions two weeks apart. Half of the patients were treated with CORDStrom and then crossed over to Placebo following a washout period and the other half were treated with Placebo and then crossed over to CORDStrom. Efficacy was assessed at 3- and 6-months from the first infusion per study arm. Thus, all patients are included in the 3- and 6-month efficacy assessment of both placebo and CORDStrom.
CORDStrom was extremely well tolerated, with no serious adverse events related to CORDStrom reported at either 3-months or 6-months post-treatment across all age and RDEB-severity patient sub-types. In children with severe disease, CORDStrom reduced itch at 3-months and led to a sustained reduction of over

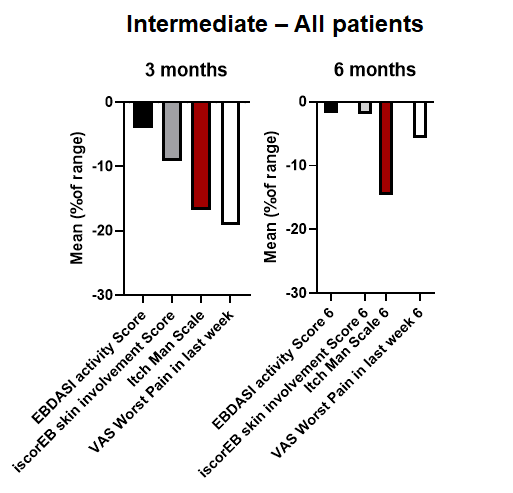
The Company and the GOSH NHS Foundation Trust entered into an exclusive commercial license whereby the Company received exclusive commercial rights to the MissionEB clinical data in exchange for (i) payment of a small initial fee, (ii) a single development milestone fee that becomes due on receipt of the first to occur marketing authorization from the FDA, EMA, or MHRA, and (iii) a commitment to supply CORDStrom to MissionEB study patients that enroll in the open label study, subject to certain limitations.
The Company participated in a Type C meeting with the FDA, the outcome of which provided information related to CMC and other regulatory topics in anticipation of the Company’s efforts to prepare and submit a BLA.
The FDA granted CORDStrom a rare pediatric disease designation (RPDD) for treatment of EB on December 13, 2024, ahead of the priority review voucher (PRV) sunset period, and as such, CORDStrom remains eligible to receive a PRV if approved by the FDA on or prior to September 30, 2026, which date may be extended by Congress. If granted, a PRV can be redeemed to receive priority review for a different product, or it may be transferred or sold.
Additionally, the FDA granted CORDStrom an orphan drug designation (ODD) on January 6th, 2025. Benefits of an ODD include certain tax credits and eligibility for select grants, waiver of FDA user fees, including the BLA application fees, access to frequent meetings with the FDA for efficient drug development, and eligibility for seven (7) years of market exclusivity post approval.
The Company intends to prepare for a pre-BLA meeting to discuss particulars of its planned BLA submission, with intent to submit a BLA in 2025 seeking approval of CORDStrom for the treatment of RDEB in pediatric patients. Concurrently, the company will also prepare to submit MAAs to the EU and UK in 2026.
“CORDStrom represents the culmination of years of dedication by members of our cell medicines R&D team to overcoming the challenges of creating a reproducible, cGMP grade MSC drug product at reasonable costs and scale to treat rare diseases like RDEB,” said Dr. Mark Lowdell, CSO of INmune Bio and inventor of CORDStrom. “The encouraging results from the blinded randomized trial in patients with intermediate and severe RDEB, combined with regulatory support and the NIHR grant, validate our approach and strengthen our resolve to deliver life-changing therapies for patients who need them most. We are heart warmed by the feedback from patients in the MissionEB study, the dedication of Dr. Anna Martinez and her group of investigators in completing this trial, all of which strengthens our resolve to seek approval for CORDStrom in pediatric RDEB and expand the indications for CORDStrom as a drug platform in the future.”
In addition to these developments involving CORDStrom, the Company reiterates plans to report top-line data on cognitive function in its MINDFuL study, a Phase II trial investigating XproTM for treatment of Alzheimer’s disease with inflammation, in June of this year, and further plans to announce additional data in its CaRe PC study, an open-label, phase I/IIa dose escalation and expansion study of INKmune in men with metastatic castration-resistant prostate cancer (mCRPC) as it becomes available throughout 2025.
The company will host a webinar at 8:30 AM ET today to discuss the results of the MissionEB study investigating CORDStrom for treatment of RDEB in pediatric patients.
Date: Monday, February 10, 2025
Time: 8:30 AM Eastern Time
Webcast: Click Here or https://lifescievents.com/event/inmunebio-2/
About CORDStrom
CORDStrom is a patent-pending cell medicine comprising aseptic, allogeneic, pooled human umbilical cord -derived mesenchymal stromal cells (hucMSCs) in suspension for injection or infusion. The CORDStrom platform leverages, among other things, proprietary screening, pooling and expansion techniques to create off-the-shelf, allogeneic, pooled hucMSCs as medicines to treat complex inflammatory diseases. CORDStrom products are designed to provide high-quality, off-the-shelf, batch-to-batch consistent, scalable, cGMP manufactured, potent cellular medicines that can be produced at low cost and with repeatable specification independent of donor characteristics. The CORDStrom product platform shares many similarities, including reagents, equipment, and procedures, with the Company’s INKmune oncology product, enabling the Company to leverage economies of scale, experienced staff, and other resources to strategically manufacture both products in a rotational campaign with resource and environmental efficiencies.
Initially developed at the INKmune manufacturing facilities utilizing UK academic grant funding, CORDStrom is an MSC product platform that shows promise as a first systemic therapy for potentially treating RDEB and many other debilitating conditions. While the first generation CORDStrom product is agnostic to disease indication, the platform enables creation of indication-specific products, which can be tuned for optimization of anti-inflammatory, immunomodulatory, wound healing, and other characteristics.
About RDEB, DDEB, EB
Epidermolysis Bullosa (EB) is a group of inherited skin disorders characterized by extreme skin fragility and blistering. Among its subtypes, Dystrophic Epidermolysis Bullosa (DEB) is notable for its division into Dominant Dystrophic EB (DDEB) and Recessive Dystrophic EB (RDEB), both caused by mutations in the COL7A1 gene which affects type VII collagen, crucial for skin adhesion. DDEB typically presents with milder symptoms, often limited to blistering on the hands, feet, elbows, and knees, with scarring, milia, and nail dystrophy being common. Conversely, RDEB is much more severe, with widespread blistering that can involve both external and internal mucous membranes, leading to significant complications like pseudosyndactyly, chronic wounds, severe scarring, and an increased risk of squamous cell carcinoma.
About INmune Bio Inc.
INmune Bio Inc. is a publicly traded (NASDAQ: INMB), clinical-stage biotechnology company focused on developing treatments that target the innate immune system to fight disease. INmune Bio has three product platforms: the Dominant-Negative Tumor Necrosis Factor (DN-TNF) product platform utilizes dominant-negative technology to selectively neutralize soluble TNF, a key driver of innate immune dysfunction and a mechanistic driver of many diseases. DN-TNF product candidates are in clinical trials to determine if they can treat Mild Alzheimer’s disease, Mild Cognitive Impairment and treatment-resistant depression (XPro™). The Natural Killer Cell Priming Platform includes INKmune® developed to prime a patient’s NK cells to eliminate minimal residual disease in patients with cancer and is currently in trials in metastatic castration-resistance prostate cancer. The third program, CORDStrom, is a proprietary pooled, allogeneic, human umbilical cord-derived mesenchymal Stromal/Stem cell (hucMSCs) platform that recently completed a blinded randomized trial in recessive dystrophic epidermolysis bullosa. INmune Bio’s product platforms utilize a precision medicine approach for diseases driven by chronic inflammation and cancer. To learn more, please visit www.inmunebio.com.
Forward Looking Statements
Clinical trials are in early stages and there is no assurance that any specific outcome will be achieved. Any statements contained in this press release that do not describe historical facts may constitute forward-looking statements as that term is defined in the Private Securities Litigation Reform Act of 1995. Any statements contained in this press release that do not describe historical facts may constitute forward-looking statements as that term is defined in the Private Securities Litigation Reform Act of 1995. Any forward-looking statements contained herein are based on current expectations but are subject to a number of risks and uncertainties. Actual results and the timing of certain events and circumstances may differ materially from those described by the forward-looking statements as a result of these risks and uncertainties. CORDStrom, XPro1595 (XPro™), and INKmune™ are still in clinical trials or preparing to start clinical trials and have not been approved by the US Food and Drug Administration (FDA) or any regulatory body and there cannot be any assurance that they will be approved by the FDA or any regulatory body or that any specific results will be achieved. The factors that could cause actual future results to differ materially from current expectations include, but are not limited to, risks and uncertainties relating to the Company’s ability to produce more drug for clinical trials; the availability of substantial additional funding for the Company to continue its operations and to conduct research and development, clinical studies and future product commercialization; and, the Company’s business, research, product development, regulatory approval, marketing and distribution plans and strategies. These and other factors are identified and described in more detail in the Company’s filings with the Securities and Exchange Commission, including the Company’s Annual Report on Form 10-K, the Company’s Quarterly Reports on Form 10-Q and the Company’s Current Reports on Form 8-K. The Company assumes no obligation to update any forward-looking statements in order to reflect any event or circumstance that may arise after the date of this release.
David Moss
Co-founder and Chief Financial Officer
(858) 964-3720
info@inmunebio.com
Daniel Carlson
Head of Investor Relations
(415) 509-4590
dcarlson@inmunebio.com








