Hyperfine, Inc. Launches AI-powered Brain Imaging Software for Enhanced Image Quality and Ease of Use
- None.
- None.
Insights
The recent CE and UKCA certifications for Hyperfine, Inc.'s eighth generation Swoop® system software mark a significant milestone in the field of medical device innovation. The integration of AI-powered features, such as diffusion-weighted imaging (DWI) AI denoising, represents a leap forward in enhancing the diagnostic capabilities of portable MRI technology. DWI is a critical component in the assessment of acute ischemic stroke, as it allows for the early detection of brain tissue affected by lack of blood flow.
From a research perspective, the improved image quality and workflow efficiencies could lead to more accurate and timely diagnoses, potentially improving patient outcomes. The ease-of-use features, like real-time patient loading assistance and the streamlined image upload process, are designed to reduce the time burden on healthcare professionals. This could translate into higher throughput in clinical settings and more rapid decision-making in acute cases. However, it is essential to monitor the long-term clinical impact of these advancements to ensure they meet safety and efficacy standards.
The expansion of Hyperfine, Inc.'s technology into international markets with the latest software's CE and UKCA approval could have significant economic implications for the healthcare sector. By providing a portable and ultra-low-field MRI solution, the Swoop® system addresses a gap in the current imaging market, potentially reducing costs associated with traditional high-field MRI systems. The portability aspect may also lead to increased access to MRI technology in remote or underserved areas, potentially reducing healthcare disparities.
Although initial costs for new technology adoption can be high, the long-term economic benefits could include reduced hospital stays and fewer repeat scans due to improved image quality. Additionally, the ability to perform MRIs at the point of care could decrease the need for patient transfers, further reducing healthcare costs. However, widespread adoption will depend on the system's cost-effectiveness compared to existing imaging solutions and the ability to integrate with current healthcare infrastructures.
Hyperfine, Inc.'s strategic move to update the Swoop® system software and obtain necessary international certifications positions the company for potential growth in the global medical imaging market. The focus on AI and improved imaging quality aligns with current market trends towards digitization and personalized healthcare. As hospitals and clinics continue to adopt advanced technologies for better patient care, Hyperfine's updated Swoop® system could see an increase in demand.
However, competition from established MRI manufacturers and regulatory hurdles in different countries could influence the pace of market penetration. It will be crucial for Hyperfine to establish strong partnerships and obtain endorsements from medical professionals to build credibility. The feedback from early adopters, as mentioned in the release, will be a valuable asset in marketing the product. Continuous innovation and responsiveness to user feedback will be key to maintaining a competitive edge in this rapidly evolving industry.
The latest software, the eighth generation of Swoop® system software, has obtained CE and UKCA approval
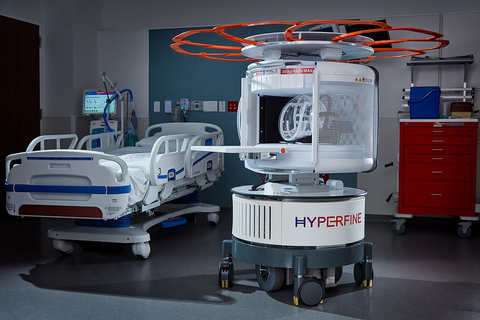
Hyperfine, Inc. has redefined brain imaging with the world’s first FDA-cleared, portable, ultra-low-field, magnetic resonance brain imaging system—the Swoop® system (Photo: Business Wire)
With CE and UKCA certifications for this latest software, Hyperfine, Inc. is well positioned for future international expansion, bringing the Swoop® system to a broader global market.
“Our latest AI-powered software, the eighth generation of our proprietary software platform, embodies our commitment to supporting clinicians in critical decision-making,” remarked Tom Teisseyre PhD, Chief Operating Officer of Hyperfine, Inc. “Our focus on image quality with this latest software has been on the DWI sequence, which is key in stroke imaging. Since its first FDA clearance in 2020, we’ve been dedicated to continually enhancing image quality and workflow efficiencies to define best-in-class, user-centric, ultra-low field MR brain imaging.”
Over 50 exams were performed in hospitals taking part in the limited market release phase, and users commented favorably on DWI image quality. Participating sites confirmed that the new streamlined upload feature materially shortened upload completion times, and the fast-positioning check was a time-saving aid and helped improve image acquisition.
“The software update on the Swoop® system has been most beneficial to our workflow. We are now able to view each series as it is being performed and can communicate with the technologist whether additional series are needed. Additionally, we can communicate with clinicians any critical findings in real-time without having to wait for the whole study to be completed," says Jennifer Villa Frabizzio MD, neuroradiologist, Radiology Group of Abington.
Hyperfine, Inc. will roll out software updates for the Swoop® system to users in the coming weeks.
For more information about the Swoop® Portable MR Imaging® system, please visit hyperfine.io.
About Hyperfine, Inc. and the Swoop® Portable MR Imaging® System
Hyperfine, Inc. (Nasdaq: HYPR) is the groundbreaking medical technology company that has redefined brain imaging with the Swoop® system—the world’s first FDA-cleared, portable, ultra-low-field, magnetic resonance brain imaging system capable of providing imaging at multiple points of care. The Swoop® system received initial
The mission of Hyperfine, Inc. is to revolutionize patient care globally through transformational, accessible, clinically relevant diagnostic imaging and data solutions. Founded by Dr. Jonathan Rothberg in a technology-based incubator called 4Catalyzer, Hyperfine, Inc. scientists, engineers, and physicists developed the Swoop® system out of a passion for redefining brain imaging methodology and how clinicians can apply accessible diagnostic imaging to patient care. Traditionally, access to costly, stationary, conventional MRI technology can be inconvenient or not available when needed most. With the portable, ultra-low-field Swoop® system, Hyperfine, Inc. is redefining the neuroimaging workflow by bringing brain imaging to the patient’s bedside. For more information, visit hyperfine.io.
Hyperfine, Swoop, and Portable MR Imaging are registered trademarks of Hyperfine, Inc.
Forward-Looking Statements
This press release includes “forward-looking statements” within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995. Actual results of Hyperfine, Inc. (the “Company”) may differ from its expectations, estimates and projections and consequently, you should not rely on these forward-looking statements as predictions of future events. Words such as “expect,” “estimate,” “project,” “budget,” “forecast,” “anticipate,” “intend,” “plan,” “may,” “will,” “could,” “should,” “believes,” “predicts,” “potential,” “continue,” and similar expressions (or the negative versions of such words or expressions) are intended to identify such forward-looking statements. These forward-looking statements include, without limitation, the Company’s goals and commercial plans, the benefits of the Company’s products and services, and the Company’s future performance and its ability to implement its strategy. These forward-looking statements involve significant risks and uncertainties that could cause the actual results to differ materially from the expected results. Most of these factors are outside of the Company’s control and are difficult to predict. Factors that may cause such differences include, but are not limited to: the success, cost and timing of the Company’s product development and commercialization activities, including the degree that the Swoop® system is accepted and used by healthcare professionals; the impact of COVID-19 on the Company’s business; the inability to maintain the listing of the Company’s Class A common stock on the Nasdaq; the Company’s inability to grow and manage growth profitably and retain its key employees; changes in applicable laws or regulations; the inability of the Company to raise financing in the future; the inability of the Company to obtain and maintain regulatory clearance or approval for its products, and any related restrictions and limitations of any cleared or approved product; the inability of the Company to identify, in-license or acquire additional technology; the inability of the Company to maintain its existing or future license, manufacturing, supply and distribution agreements and to obtain adequate supply of its products; the inability of the Company to compete with other companies currently marketing or engaged in the development of products and services that the Company is currently marketing or developing; the size and growth potential of the markets for the Company’s products and services, and its ability to serve those markets, either alone or in partnership with others; the pricing of the Company’s products and services and reimbursement for medical procedures conducted using the Company’s products and services; the Company’s estimates regarding expenses, revenue, capital requirements and needs for additional financing; the Company’s financial performance; and other risks and uncertainties indicated from time to time in Company’s filings with the Securities and Exchange Commission, including those under “Risk Factors” therein. The Company cautions readers that the foregoing list of factors is not exclusive and that readers should not place undue reliance upon any forward-looking statements, which speak only as of the date made. The Company does not undertake or accept any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements to reflect any change in its expectations or any change in events, conditions or circumstances on which any such statement is based.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240109012126/en/
Media Contact
Shay Smith
Health+Commerce
Shay@healthandcommerce.com
Investor Contact
Marissa Bych
Gilmartin Group LLC
marissa@gilmartinir.com
Source: Hyperfine, Inc.







