Exscientia Reports Topline Data From EXS-21546 Phase 1a Study Demonstrating Targeted A2A Receptor Signaling Inhibition in Healthy Volunteers
Exscientia announced positive results from its Phase 1 healthy volunteer study of EXS-21546, a selective A2A receptor antagonist. The study confirmed potency and selectivity with low CNS exposure, supporting a Phase 1b/2 study in patients with high adenosine signature solid tumors, anticipated to start in the second half of 2022. The trial showed dose-dependent effects and was well-tolerated, with only one serious adverse event recorded that resolved without intervention. These findings enhance the potential for EXS-21546 to improve immunomodulatory treatments in cancer therapy.
- Topline data confirm potency and selectivity of EXS-21546.
- No CNS adverse events reported during trials.
- Phase 1b/2 study set to begin in the second half of 2022.
- Dose-dependent inhibition of CREB phosphorylation observed.
- One Grade 3 Serious Adverse Event reported in the MAD portion of the trial.
Insights
Analyzing...
Clinical data builds upon body of evidence suggesting EXS-21546 is a highly potent and selective A2AR antagonist with low CNS exposure
Ongoing translational work to establish predictive biomarker to enable targeting of patients most likely to benefit from EXS-21546
(Graphic: Business Wire)
“Topline data from our phase 1a study of EXS-21546 demonstrate the ability of our AI-based platform to create novel molecules based upon defined design objectives and with a high level of translatability to human biology. EXS-21546 is a pilot programme from the early days of our platform, and we are proud that it achieved our targeted objectives of potency, selectivity and pharmacokinetics,” said
The EXS-21546 phase 1a study was a three-part dose-finding trial evaluating the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of single ascending doses (SAD) and multiple ascending doses (MAD) of EXS-21546. The study randomised 60 healthy male subjects across all three parts. One of the study’s primary goals was to inform the optimal starting dose for EXS-21546 for the Phase 1b/2 study in cancer patients.
The study showed that observed human PK for EXS-21546 was in line with what had been designed for and predicted in preclinical modeling, supporting a twice-daily (BID) dose for continuous A2A receptor inhibition over a dosing interval.
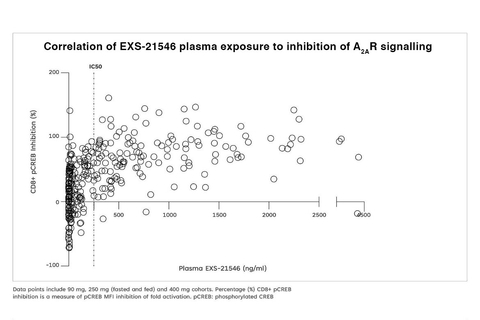
EXS-21546 showed dose-dependent inhibition of CREB phosphorylation in CD8-positive cells, with the PD profile mirroring plasma exposure. Inhibition of A2A receptor signaling was sustained over the BID dosing period, demonstrating a level of lasting target engagement.
EXS-21546 was well-tolerated with no CNS adverse events reported in the SAD portion at all doses (30mg, 90mg, 250mg, 400mg) and in the MAD portion at 150mg BID. In the MAD phase, a lab abnormality of elevated ALT and AST was observed in one subject that was classified as a Grade 3 Serious Adverse Event. This event was observed in one subject three days after completion of 14 days of treatment and resolved without medical intervention. The subject was asymptomatic during the treatment period and reported no adverse events while on drug.
Based on these results,
The Company expects to share additional data from this Phase 1a study at future medical meetings.
About EXS-21546
EXS-21546, an AI-designed A2A receptor antagonist, was co-invented and developed by
Some tumours produce high levels of adenosine, which binds and activates A2A receptors on immune cells, resulting in the suppression of the anti-tumour activity of the immune system. EXS-21546 is being investigated for its ability to prevent high concentrations of adenosine from activating the A2A receptor, and thereby its potential to promote the anti-tumour activity of the immune cells.
About the EXS-21546 Phase 1a Trial
The Phase 1 study was a three-part study in male healthy volunteers to assess the safety, tolerability, pharmacokinetics and pharmacodynamics (PK/PD) of EXS-21546. Part 1 was a randomised, double-blind, placebo-controlled, SAD study with a food effect assessment where 41 healthy volunteers were randomised 3:1 (in a ratio of 6 active to 2 placebo per cohort). Part 2 was a randomised, double-blind, placebo controlled, MAD study over 14 days. Part 2 was completed after the enrolment of 1 cohort (8 subjects) who received 150mg EXS-21546 BID. Part 3 was a 3-way crossover, open label, randomised study, where 11 subjects were enrolled to evaluate a capsule formulation (fed and fasted) as compared to an oral suspension (fasted) formulation.
About
Visit us at https://www.exscientia.ai or follow us on Twitter @exscientiaAI.
Forward-Looking Statements
This press release contains certain forward-looking statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995, including statements with regard to Exscientia’s expectations with respect to the progress of development of candidate molecules, timing and progress of, and data reported from, preclinical studies and clinical trials of Exscientia’s product candidates, and Exscientia’s expectations regarding its precision medicine platform and AI-driven drug discovery platform. Words such as “anticipates,” "believes," “expects,” "intends," "projects," "anticipates," and "future" or similar expressions are intended to identify forward-looking statements. These forward-looking statements are subject to the uncertainties inherent in predicting future results and conditions, including the scope, progress and expansion of Exscientia’s product development efforts; the initiation, scope and progress of Exscientia’s and its partners’ clinical trials and ramifications for the cost thereof; clinical, scientific, regulatory and technical developments; and those inherent in the process of discovering, developing and commercialising product candidates that are safe and effective for use as human therapeutics, and in the endeavor of building a business around such product candidates.
View source version on businesswire.com: https://www.businesswire.com/news/home/20220614005457/en/
Investors:
investors@exscientia.ai
Media:
media@exscientia.ai
Source: