Exscientia Business Update for Third Quarter 2021
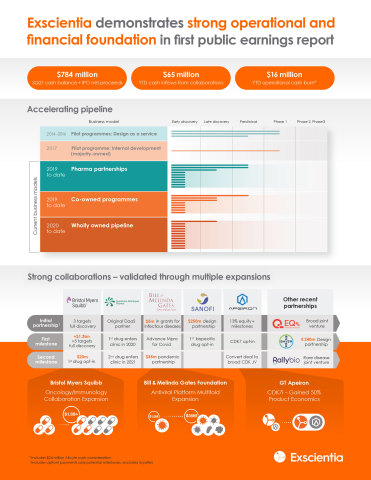
Exscientia (Nasdaq: EXAI) reported significant advancements in its pipeline and collaborations, alongside financial results for Q3 2021. Notably, the company achieved $31.3 million in revenue, a substantial increase from $7.7 million in the same period last year. Key developments include expanded partnerships with Bristol Myers Squibb and the Bill & Melinda Gates Foundation, as well as the integration of Allcyte's tissue platform. Exscientia's cash position at Q3 was $783.7 million, bolstered by a $510.4 million financing. The firm continues to invest in expanding its drug discovery labs and aims for cash inflows of $75.0-$85.0 million for 2021.
- Q3 2021 revenue increased to $31.3 million from $7.7 million in Q3 2020.
- Expanded collaboration with Bristol Myers Squibb could exceed $1.3 billion in value.
- Acquisition of Allcyte enhances drug discovery capabilities.
- Strong cash position of $783.7 million post IPO financing.
- Operating loss increased to $6.9 million in Q3 2021 from $9.2 million in Q3 2020.
- General and administrative expenses rose to $26.1 million, representing 34% of total operating expenses.
(Graphic: Business Wire)
Recent advancements in the Company’s pipeline, collaborations and operations as well as financial results for the third quarter ended
Highlights
Leveraging deep learning on patient tissue samples at scale to transform target discovery and patient selection
- Expanded investigation of patient tissue models in solid tumours, including ongoing analysis of EXS-617 in ovarian and breast cancer models
- Published EXALT-1 clinical trial results in Cancer Discovery that highlighted benefit of the first AI-supported functional precision medicine platform to guide treatment selection and improve outcomes in patients with advanced haematological cancer
- Completed acquisition of Allcyte, integrating leading tissue platform into Exscientia’s end-to-end drug discovery operations
Pipeline progress marked by candidate nomination, significant expansion of existing collaborations
- Partnered programmes
-
Expanded Bristol Myers Squibb collaboration to include potential targets across immunology and oncology; potential deal value beyond
$1.3 billion -
BMS in-licensed first candidate from original 2019 collaboration, a potential first-in-class immunology medicine, triggering
$20.0 million
-
BMS in-licensed first candidate from original 2019 collaboration, a potential first-in-class immunology medicine, triggering
- Co-owned programmes
- EXS-617: development candidate nominated for CDK7 inhibitor from expanded joint venture with GT Apeiron; additional translational data anticipated 1H 2022
- First two targets selected in EQRx joint venture
- Wholly and majority owned programmes
- EXS-21546: Phase 1 data for A2a antagonist in high adenosine signature cancers expected 1H 2022; translational research programmes for patient selection ongoing
-
Entered into third collaboration with
Bill & Melinda Gates Foundation that added portfolio of up to five antiviral small molecule drug candidates and accelerated development of SARS-CoV-2 lead programme
Strengthened balance sheet providing several years of cash runway to deliver AI-designed molecules and accelerate pipeline growth
- Expect between
- In October, closed
- Cash at the end of the third quarter 2021, adjusted to include the net proceeds from the initial public offering completed on
Expanding drug discovery and automation laboratories to scale capacity and operations
- Opened 21,000-square foot expansion of facilities at
- Building 26,000-square foot laboratory in
- Building 50,000-square foot precision medicine centre of excellence in
- Added to
“Exscientia is leading the modernisation of how new medicines are created by using AI in each step of discovery and development. Demonstrating our unique translational capabilities, we recently published groundbreaking results showing our AI precision medicine platform can improve outcomes for cancer patients. We have also propelled our pipeline through substantial investment in our own new medicine programmes, significantly expanded our relationship with Bristol Myers Squibb, and added a portfolio of pandemic preparedness antivirals to our work with the
Investor Call and Webcast Information
Third Quarter Financial Results
For the convenience of the reader, the Company has translated pound sterling amounts to
Revenue: Revenue for the nine months ended
R&D and cost of drug discovery: Due to various collaboration structures, R&D expenses may be included under multiple accounting line items. The tables below show how these expenses are separated across the accounting categories.
Three months ended
|
COGS |
R&D |
Share of JV loss |
Total |
Partnered Programmes |
6.5 |
–– |
–– |
6.5 |
Co-owned Programmes |
–– |
1.0 |
0.3 |
1.4 |
Internal Pipeline and |
–– |
16.4 |
–– |
16.4 |
Total |
6.5 |
17.4 |
0.3 |
24.2 |
Nine months ended
|
COGS |
R&D |
Share of JV loss |
Total |
Partnered Programmes |
16.6 |
–– |
–– |
16.6 |
Co-owned Programmes |
–– |
1.8 |
1.3 |
3.1 |
Internal Pipeline and |
–– |
32.3 |
–– |
32.3 |
Total |
16.6 |
34.1 |
1.3 |
52.0 |
General and administrative expenses: G&A expenses for the nine months ended
Financial Outlook for 2021 & 2022
For the first three quarters of 2021,
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS (unaudited)
(in £ millions, except per share data)
|
Three months ended
|
Nine months ended
|
||
|
2021 |
2020 |
2021 |
2020 |
Revenue |
17.6 |
1.0 |
23.2 |
5.7 |
Cost of sales |
(4.8) |
(3.3) |
(12.3) |
(10.3) |
Research and development expenses |
(12.9) |
(2.9) |
(25.3) |
(7.2) |
General and administrative expenses |
(8.6) |
(1.5) |
(19.4) |
(4.4) |
Operating expenses |
(26.3) |
(7.7) |
(57.0) |
(21.8) |
Foreign exchange gains/(losses) |
1.7 |
(2.4) |
(1.2) |
(0.9) |
Other income |
1.8 |
0.4 |
3.0 |
0.8 |
Operating loss |
(5.3) |
(8.7) |
(32.0) |
(16.2) |
Finance income/(expense) |
(0.1) |
(0.0) |
(0.1) |
0.0 |
Share of loss on joint ventures |
(0.2) |
(0.4) |
(1.0) |
(0.9) |
Loss on derivative financial instruments |
(1.4) |
–– |
–– |
–– |
Loss before taxation |
(6.9) |
(9.2) |
(33.1) |
(17.0) |
Income tax benefit |
1.9 |
0.8 |
4.0 |
1.5 |
Loss for the period |
(5.1) |
(8.4) |
(29.1) |
(15.6) |
Net loss per share |
(0.06) |
(0.08) |
(0.32) |
(0.15) |
CONSTANT CURRENCY CONVERSION (unaudited)
(in $ millions, except per share data, at the rate of
|
Three months ended
|
Nine months ended
|
||
|
2021 |
2020 |
2021 |
2020 |
Revenue |
23.8 |
1.3 |
31.3 |
7.7 |
Cost of sales |
(6.5) |
(4.5) |
(16.6) |
(13.8) |
Research and development expenses |
(17.4) |
(3.8) |
(34.1) |
(9.7) |
General and administrative expenses |
(11.6) |
(2.0) |
(26.1) |
(6.0) |
Operating expenses |
(35.5) |
(10.4) |
(76.8) |
(29.4) |
Foreign exchange gains/(losses) |
2.2 |
(3.2) |
(1.7) |
(1.2) |
Other income |
2.4 |
0.5 |
4.0 |
1.1 |
Operating loss |
(7.1) |
(11.7) |
(43.1) |
(21.8) |
Finance income/(expense) |
(0.1) |
(0.0) |
(0.2) |
0.0 |
Share of loss on joint ventures |
(0.3) |
(0.6) |
(1.3) |
(1.2) |
Loss on derivative financial instruments |
(1.8) |
–– |
–– |
–– |
Loss before taxation |
(9.3) |
(12.3) |
(44.6) |
(22.9) |
Income tax benefit |
2.5 |
1.1 |
5.3 |
2.0 |
Loss for the period |
(6.8) |
(11.3) |
(39.3) |
(21.0) |
Net loss per share |
(0.08) |
(0.11) |
(0.43) |
(0.20) |
About
Forward-Looking Statements
This press release contains forward-looking statements as that term is defined in the Private Securities Litigation Reform Act of 1995, including with respect to the progress of development of candidate molecules, timing and progress of, and data reported from, clinical trials of Exscientia’s product candidates, and Exscientia’s expectations regarding its projected revenue and cash runway. Any statement describing Exscientia’s goals, expectations, financial or other projections, intentions or beliefs is a forward-looking statement and should be considered an at-risk statement. Such statements are subject to a number of risks, uncertainties and assumptions, including those related to the impact that the COVID-19 pandemic could have on the Company’s business, and including the scope, progress and expansion of Exscientia’s product development efforts; the initiation, scope and progress of Exscientia’s and its partners’ clinical trials and ramifications for the cost thereof; clinical, scientific, regulatory and technical developments; and those inherent in the process of discovering, developing and commercialising product candidates that are safe and effective for use as human therapeutics, and in the endeavor of building a business around such product candidates. In light of these risks and uncertainties, and other risks and uncertainties that are described in the Risk Factors section and other sections of Exscientia’s Registration Statement on Form F-1, filed with the
View source version on businesswire.com: https://www.businesswire.com/news/home/20211117006300/en/
Investors:
investors@exscientia.ai
Media:
media@exscientia.ai
Source:
FAQ
What were Exscientia's Q3 2021 financial results?
What collaborations did Exscientia announce in Q3 2021?
What is Exscientia’s cash position as of Q3 2021?
What is the expected cash inflow for Exscientia in 2021?