Exscientia Business Update for Fourth Quarter and Full Year 2021
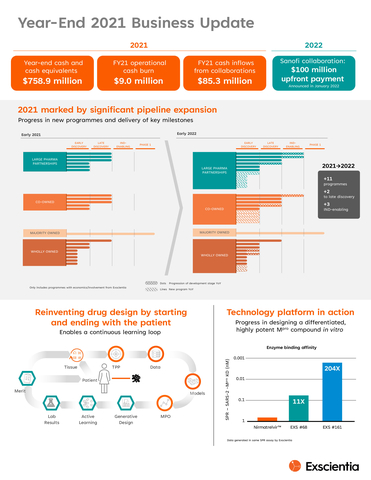
Exscientia plc (Nasdaq: EXAI) reported significant advancements in its pipeline and financial results for 2021. Key highlights include 11 new programmes added, a $100 million upfront payment from Sanofi focused on oncology and immunology, and the achievement of preclinical milestones in collaborations with Bayer and Bristol Myers Squibb. Financially, the company generated $36.9 million in revenue, a $23.9 million increase year-over-year, with $85.3 million cash flow from collaborations. As of year-end, cash reserves stood at $758.9 million, positioning the company for future growth.
- Revenue increased to $36.9 million, up $23.9 million year-over-year.
- Secured $100 million upfront payment from Sanofi collaboration.
- Achieved preclinical proof of concept milestone with Bayer.
- Added 11 new drug programmes, advancing three into IND-enabling studies.
- Net loss for 2021 was $66.5 million, increasing from $30.1 million in 2020.
- Operating loss of $74.1 million for the year.
- General and administrative expenses grew by $26.9 million.
Insights
Analyzing...
(Graphic: Business Wire)
Recent advancements in the Company’s pipeline, collaborations and operations as well as financial results for the fourth quarter and full year 2021 are summarised below. In addition,
Recent Highlights
Progressed pipeline led by significant collaborations, expansion, and programme advancement
- Year-over-year pipeline progress with 11 new programmes added, including advancement of three programmes into IND-enabling studies and two programmes to late discovery
- Partnered programmes
-
Established collaboration with Sanofi in
January 2022 focused on development of up to 15 novel small molecule candidates across oncology and immunology, received$100 million $5.2 billion - Commenced eighth drug discovery project with Bristol Myers Squibb (BMS) in early 2022 for programme against an undisclosed oncology target
- Achieved preclinical proof of concept milestone for a target within Bayer collaboration supporting advancement into late discovery
- Co-owned programmes
-
Entered into IND-enabling studies for GTAEXS-617, a CDK7 inhibitor co-owned with GT Apeiron; IND/CTA submission expected by year-end 2022; additional translational data in several tumour types to be presented at the
April 2022 American Association of Cancer Research (AACR) Annual Meeting and throughout 2022 - Oncology target selected with EQRx, the third in the multi-target collaboration
- Wholly and majority owned programmes
- Phase 1 healthy volunteer top-line data for EXS-21546, A2a antagonist in high adenosine signature cancers, on track for the first half of 2022; translational research on identifying adenosine specific gene signatures for patient selection to be presented at AACR 2022
-
With support from the
Bill & Melinda Gates Foundation ,Exscientia has designed an Mpro molecule with pan-coronavirus activity demonstrating 200x greater potency in vitro than a commercially available oral COVID-19 antiviral; ongoing compound development with candidate nomination anticipated in the second half of 2022
Balanced business model has generated meaningful cash flows and strong balance sheet
-
- 2021 net cash outflows from operations of approximately
-
Peer-reviewed publications and upcoming scientific meeting presence showcase impact of translational research, clinical impact of precision medicine platform and differentiated technology platform
- Three abstracts accepted for poster presentation at AACR 2022 highlight the potential of
- EXALT-1 clinical trial results published in Cancer Discovery demonstrate the benefit of the first AI-supported functional precision medicine platform to guide treatment selection and improve outcomes in patients with advanced haematological cancers
- Select peer-reviewed publications describe recent advancements in
- Fragment Hotspot Mapping to Identify Selectivity-Determining Regions Between Related Proteins describes a computational method to map protein binding pockets and identify critical regions that can be exploited to generate selective molecules
- Deep generative design with 3D pharmacophoric constraints demonstrates the potential of a new method (DEVELOP) to utilise 3D representations of molecules and generate compounds with improved properties over previous methods
- Generating property-matched decoy molecules using deep learning showcases a deep learning method for generating molecules to validate and improve virtual screening methods
Strengthened team to position
-
- Professor
- Significant progress in building out clinical organisation, including clinical operations, to prepare for pipeline expansion and future clinical trials
- Experienced drug discovery team established in
“2021 was a transformational year for
Investor Call and Webcast Information
Fourth Quarter and Full Year 2021 Financial Results
For the convenience of the reader, the Company has translated pound sterling amounts to
Revenue: Revenue for the full year 2021, was
R&D and cost of drug discovery: Due to various collaboration structures, R&D expenses may be included under multiple accounting line items. The tables below show how these expenses are separated across the accounting categories.
Three months ended
|
COGS |
R&D |
Share of
|
Total |
||||
Partnered Programmes |
6.5 |
- |
- |
6.5 |
||||
Co-owned Programmes |
- |
2.5 |
0.2 |
2.7 |
||||
Internal Pipeline and |
- |
22.8 |
- |
22.8 |
||||
Total |
6.5 |
25.3 |
0.2 |
32.0 |
Twelve months ended
|
COGS |
R&D |
Share of
|
Total |
||||
Partnered Programmes |
23.1 |
- |
- |
23.1 |
||||
Co-owned Programmes |
- |
4.2 |
1.6 |
5.8 |
||||
Internal Pipeline and |
- |
55.3 |
- |
55.3 |
||||
Total |
23.1 |
59.5 |
1.6 |
84.1 |
General and administrative expenses: G&A expenses for the three months ended
Cash inflows: For the full year 2021,
Cash and cash equivalents: Cash and cash equivalents as of
SELECTED CONSOLIDATED STATEMENT OF OPERATIONS, CONSTANT CURRENCY CONVERSION (unaudited)
($ millions, except per share data, at the rate of
|
Three months ended
|
Twelve months ended
|
||
|
2021 |
2020 |
2021 |
2020 |
Revenue |
5.6 |
5.3 |
37.0 |
13.1 |
Cost of sales |
(6.5) |
(5.4) |
(23.1) |
(19.2) |
Research and development expenses |
(25.3) |
(5.0) |
(59.5) |
(14.7) |
General and administrative expenses |
(8.6) |
(1.9) |
(34.8) |
(7.9) |
Operating expenses |
(40.4) |
(12.4) |
(117.4) |
(41.9) |
Foreign exchange gains/(losses) |
2.9 |
(2.9) |
1.3 |
4.1 |
Other income |
1.0 |
0.5 |
5.1 |
1.6 |
Operating loss |
(30.9) |
(9.5) |
(74.1) |
(31.3) |
Finance income/(expense) |
(0.0) |
(0.0) |
(0.2) |
0.0 |
Share of loss on joint ventures |
(0.2) |
(0.5) |
(1.6) |
(1.6) |
Loss before taxation |
(31.2) |
(9.9) |
(75.9) |
(32.9) |
Income tax benefit |
4.0 |
0.9 |
9.4 |
2.8 |
Loss for the period |
(27.1) |
(9.1) |
(66.5) |
(30.1) |
Net loss per share |
(0.91) |
(0.56) |
(1.33) |
(0.98) |
SELECTED CONSOLIDATED BALANCE SHEET, CONSTANT CURRENCY CONVERSION (unaudited)
($ millions, except per share data, at the rate of
|
|
|
Cash and cash equivalents |
758.9 |
84.5 |
Total assets |
864.9 |
104.9 |
Total equity |
765.2 |
79.0 |
Total liabilities |
99.7 |
25.9 |
Total equity and liabilities |
864.9 |
104.9 |
SELECTED CONSOLIDATED STATEMENT OF CASH FLOWS. CONSTANT CURRENCY CONVERSION (unaudited)
($ millions, except per share data, at the rate of
|
|
|
Net cash flows used in operating activities |
(9.0) |
(28.9) |
Net cash flows used in investing activities |
(35.9) |
(5.1) |
Net cash generated from financing activities |
719.5 |
76.0 |
Net increase in cash and cash equivalents |
674.6 |
42.0 |
About
Forward-Looking Statements
This press release contains forward-looking statements as that term is defined in the Private Securities Litigation Reform Act of 1995, including with respect to the progress of discovery and development of candidate molecules, and the timing and progress of, and data reported from, clinical trials of Exscientia’s product candidates, and Exscientia’s expectations regarding its projected revenue and cash runway. Any statement describing Exscientia’s goals, expectations, financial or other projections, intentions or beliefs is a forward-looking statement and should be considered an at-risk statement. Such statements are subject to a number of risks, uncertainties and assumptions, including those related to the impact that the COVID-19 pandemic could have on the Company’s business, and including the scope, progress and expansion of Exscientia’s product development efforts; the initiation, scope and progress of Exscientia’s and its partners’ clinical trials and ramifications for the cost thereof; clinical, scientific, regulatory and technical developments; and those inherent in the process of discovering, developing and commercialising product candidates that are safe and effective for use as human therapeutics, and in the endeavor of building a business around such product candidates. In light of these risks and uncertainties, and other risks and uncertainties that are described in the Risk Factors section and other sections of Exscientia’s Registration Statement on Form F-1, filed with the
View source version on businesswire.com: https://www.businesswire.com/news/home/20220323005753/en/
Investors:
investors@exscientia.ai
Media:
media@exscientia.ai
Source: