Cytokinetics Announces Data From Phase 1 Study of CK-4021586
Cytokinetics (Nasdaq: CYTK) announced positive results from the Phase 1 study of CK-4021586 (CK-586), a cardiac myosin inhibitor for potential treatment of heart failure with preserved ejection fraction (HFpEF). The study met its primary and secondary objectives, demonstrating safety, tolerability, and favorable pharmacokinetics of CK-586. Key findings include:
- No serious adverse events observed
- Half-life of 14-17 hours
- Dose-linearity without change in half-life
- Predictable PK/PD relationship
- Mean decrease in LVEF <5% at highest dose
These results support advancement to a Phase 2 clinical trial in HFpEF patients, expected to begin in Q4 2024.
Cytokinetics (Nasdaq: CYTK) ha annunciato risultati positivi dallo studio di Fase 1 di CK-4021586 (CK-586), un inibitore della miosina cardiaca per il trattamento potenziale dell'insufficienza cardiaca con frazione di eiezione preservata (HFpEF). Lo studio ha raggiunto i suoi obiettivi primari e secondari, dimostrando la sicurezza, la tollerabilità e la farmacocinetica favorevole di CK-586. I risultati chiave includono:
- Nessun evento avverso grave osservato
- Emivita di 14-17 ore
- Linearità della dose senza cambiamento dell'emivita
- Relazione PK/PD prevedibile
- Riduzione media della LVEF <5% alla dose più alta
Questi risultati supportano il passaggio a un trial clinico di Fase 2 in pazienti con HFpEF, previsto per iniziare nel Q4 2024.
Cytokinetics (Nasdaq: CYTK) anunció resultados positivos del estudio de Fase 1 de CK-4021586 (CK-586), un inhibidor de miosina cardíaca para el tratamiento potencial de la insuficiencia cardíaca con fracción de eyección preservada (HFpEF). El estudio cumplió con sus objetivos primarios y secundarios, demostrando la seguridad, tolerabilidad y farmacocinética favorable de CK-586. Los hallazgos clave incluyen:
- No se observaron eventos adversos graves
- Vida media de 14-17 horas
- Linealidad de dosis sin cambio en la vida media
- Relación PK/PD predecible
- Disminución media en LVEF <5% en la dosis más alta
Estos resultados respaldan el avance a un ensayo clínico de Fase 2 en pacientes con HFpEF, que se espera comience en el Q4 de 2024.
Cytokinetics (Nasdaq: CYTK)는 심부전율 보존(HEpEF) 가능한 치료를 위한 심장 미오신 억제제 CK-4021586 (CK-586)의 1상 연구에서 긍정적인 결과를 발표했습니다. 이 연구는 안전성, 내약성, CK-586의 유리한 약리학적 성질을 입증하면서 주요 및 부차적 목표를 달성했습니다. 주요 발견 사항은 다음과 같습니다:
- 심각한 이상 반응이 관찰되지 않음
- 반감기 14-17시간
- 반감기 변화 없이 용량 선형성
- 예측 가능한 PK/PD 관계
- 최고 용량에서 LVEF 평균 감소 <5%
이러한 결과는 2024년 4분기에 시작될 기대되는 HFpEF 환자를 대상으로 하는 2상 임상 시험으로의 발전을 지지합니다.
Cytokinetics (Nasdaq: CYTK) a annoncé des résultats positifs de l'étude de Phase 1 de CK-4021586 (CK-586), un inhibiteur de myosine cardiaque pour le traitement potentiel de l'insuffisance cardiaque avec fraction d'éjection préservée (HFpEF). L'étude a atteint ses objectifs principaux et secondaires, démontrant la sécurité, la tolérabilité et la pharmacocinétique favorable de CK-586. Les résultats clés incluent:
- Aucun événement indésirable grave observé
- Une demi-vie de 14-17 heures
- Linéarité de la dose sans changement de la demi-vie
- Relation PK/PD prévisible
- Diminution moyenne de la LVEF <5% à la dose la plus élevée
Ces résultats soutiennent le passage à un essai clinique de Phase 2 chez des patients atteints d'HFpEF, prévu pour débuter au T4 2024.
Cytokinetics (Nasdaq: CYTK) gab positive Ergebnisse aus der Phase-1-Studie von CK-4021586 (CK-586) bekannt, einem Hemmstoff der Herzmiosin zur potenziellen Behandlung von Herzinsuffizienz mit erhaltener Ejektionsfraktion (HFpEF). Die Studie erreichte ihre primären und sekundären Ziele und zeigte die Sicherheit, Verträglichkeit und günstige Pharmakokinetik von CK-586. Wichtige Ergebnisse umfassen:
- Keine schwerwiegenden unerwünschten Ereignisse beobachtet
- Halbwertszeit von 14-17 Stunden
- Dosish Linearität ohne Veränderung der Halbwertszeit
- Vorhersagbare PK/PD-Beziehung
- Mittlerer Rückgang der LVEF <5% bei der höchsten Dosis
Diese Ergebnisse unterstützen den Übergang zu einem Phase-2-Klinikversuch an HFpEF-Patienten, der für das 4. Quartal 2024 erwartet wird.
- Phase 1 study of CK-586 met primary and secondary objectives
- CK-586 demonstrated safety and tolerability in healthy participants
- Favorable pharmacokinetic profile with 14-17 hour half-life
- Predictable and shallow PK/PD relationship observed
- Results support advancement to Phase 2 clinical trial
- Phase 2 trial in HFpEF patients expected to begin in Q4 2024
- None.
Insights
The Phase 1 study results for CK-4021586 (CK-586) are promising for Cytokinetics. The drug, a cardiac myosin inhibitor, demonstrated safety and tolerability in healthy participants with no serious adverse events. Key pharmacokinetic findings include:
- Half-life of 14-17 hours
- Dose-linearity without changes in half-life
- Steady-state reached within 7 days
The shallow and predictable PK/PD relationship suggests a once-daily fixed dosing regimen may be feasible. The observed decrease in LVEF (<
This Phase 1 data represents a significant milestone for Cytokinetics, potentially expanding their pipeline in the lucrative cardiovascular market. The planned Phase 2 trial in Q4 2024 for HFpEF patients is a key catalyst to watch. If successful, CK-586 could address a substantial market opportunity, as HFpEF affects about half of all heart failure patients with treatment options. However, investors should note that:
- Phase 2 results are important for validating efficacy in the target population
- Competition in the HFpEF space is intensifying
- Timeline to potential commercialization is still several years away
While positive, this early-stage data doesn't guarantee clinical or commercial success. Cytokinetics' financial position and ability to fund further development will be critical factors to monitor.
Phase 2 Clinical Trial in Patients with Heart Failure with Preserved Ejection Fraction Expected to Begin in Q4 2024
SOUTH SAN FRANCISCO, Calif., Sept. 09, 2024 (GLOBE NEWSWIRE) -- Cytokinetics, Incorporated (Nasdaq: CYTK) today announced that data from the Phase 1 study of CK-4021586 (CK-586) were presented in a poster session at the American College of Clinical Pharmacology (ACCP) Annual Meeting in Bethesda, MD. The study met its primary and secondary objectives to assess the safety, tolerability and pharmacokinetics (PK) of single and multiple oral doses of CK-586. The data support the advancement of CK-586 to a Phase 2 clinical trial in patients with heart failure with preserved ejection fraction (HFpEF) which is expected to begin in Q4 2024. CK-586 is a cardiac myosin inhibitor in development for the potential treatment of a subgroup of patients with symptomatic HFpEF with hypercontractility and ventricular hypertrophy.
“The results from this Phase 1 study replicate pre-clinical findings that show CK-586 directly reduces cardiac contractility at the level of the sarcomere. Importantly, CK-586 was observed to have a shallow and predictable PK/PD relationship and half-life that enables a once-daily fixed dosing regimen in patients with HFpEF,” said Stuart Kupfer, M.D., Senior Vice President, Chief Medical Officer. “Preparations are underway for a Phase 2 clinical trial of CK-586 in a subset of patients with HFpEF that we plan to start in the fourth quarter.”
Phase 1 Design and Key Findings
The primary objective of this Phase 1 double-blind randomized, placebo-controlled, single and multiple ascending dose clinical study was to evaluate the safety, tolerability and PK of CK-586 when administered orally to healthy participants. The study design included seven single ascending dose cohorts (10 mg to 600 mg) comprised of 10 participants each, and two multiple-dose cohorts (100 and 200 mg once daily) comprised of 10 participants each.
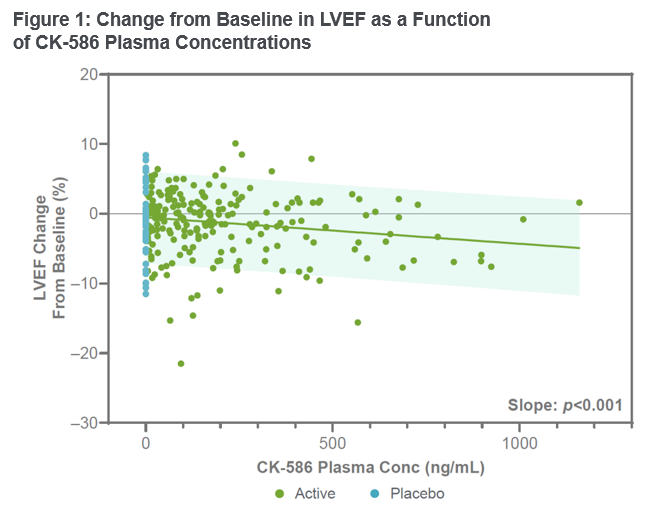
This study data demonstrated that CK-586 was safe and well tolerated in healthy participants. No serious adverse events were observed and the stopping criteria for the study were not met. The half-life of CK-586 was observed to be in the range of 14 to 17 hours. CK-586 demonstrated dose-linearity without a change in half-life over a wide range of exposures, with steady state appearing evident within seven days of dosing. Left ventricular ejection fraction (LVEF) and left ventricular fractional shortening (LVFS) decreased from baseline in an exposure-dependent manner, and the pharmacokinetic/pharmacodynamic (PK/PD) relationship appeared shallow and predictable (Figure 1). At the highest single dose of 600 mg, the mean decrease in LVEF was <
About CK-4021586 (CK-586)
CK-4021586 (CK-586) is a novel, selective, oral, small molecule cardiac myosin inhibitor designed to reduce the hypercontractility associated with heart failure with preserved ejection fraction (HFpEF). CK-586 selectively inhibits the ATPase of intact cardiac myosin but does not inhibit the ATPase of subfragment-1 of myosin (S1) as does aficamten, a cardiac myosin inhibitor also developed by the Company. Unlike aficamten, the inhibitory effect of CK-586 requires the presence of the regulatory light chain (RLC) of myosin in the context of the intact myosin dimer (heavy meromyosin or HMM). In preclinical models, CK-586 reduced cardiac hypercontractility by decreasing the number of active myosin cross-bridges during cardiac contraction thereby reducing the contractile force, without effect on calcium transients. In engineered human HCM heart tissues, CK-586 demonstrated a shallow force-concentration response and improved lusitropy. Lending support for investigating this mechanism of action in HFpEF, a subset of patients with HFpEF resemble patients with non-obstructive hypertrophic cardiomyopathy (HCM) in that those patients have higher ejection fractions, thickened walls of their heart, elevated biomarkers, and symptoms of heart failure. Data from a Phase 2 clinical trial of aficamten in patients with non-obstructive HCM show that aficamten was well tolerated, improved patient reported outcomes (Kansas City Cardiomyopathy Questionnaire (KCCQ) and New York Heart Association (NYHA) Functional Class) and biomarkers, measures that are also relevant to HFpEF.
About Heart Failure with Preserved Ejection Fraction
Heart failure is a grievous condition that affects more than 64 million people worldwide.1 Approximately 6.7 million Americans have heart failure, which is expected to increase to over 8.5 million Americans by 2030.2 Approximately half of patients with heart failure have heart failure with preserved ejection fraction (HFpEF)3, and the prevalence of HFpEF is increasing.2,4 A subset of HFpEF patients with hypercontractility, ventricular hypertrophy, elevated biomarkers and symptoms of heart failure may benefit from treatment with a cardiac sarcomere inhibitor. Approximately
About Cytokinetics
Cytokinetics is a late-stage, specialty cardiovascular biopharmaceutical company focused on discovering, developing and commercializing muscle biology-directed drug candidates as potential treatments for debilitating diseases in which cardiac muscle performance is compromised. As a leader in muscle biology and the mechanics of muscle performance, the company is developing small molecule drug candidates specifically engineered to impact myocardial muscle function and contractility. Cytokinetics is preparing for regulatory submissions for aficamten, its next-in-class cardiac myosin inhibitor, following positive results from SEQUOIA-HCM, the pivotal Phase 3 clinical trial in obstructive hypertrophic cardiomyopathy which were published in the New England Journal of Medicine. Aficamten is also currently being evaluated in MAPLE-HCM, a Phase 3 clinical trial of aficamten as monotherapy compared to metoprolol as monotherapy in patients with obstructive HCM, ACACIA-HCM, a Phase 3 clinical trial of aficamten in patients with non-obstructive HCM, CEDAR-HCM, a clinical trial of aficamten in a pediatric population with obstructive HCM, and FOREST-HCM, an open-label extension clinical study of aficamten in patients with HCM. Cytokinetics is also developing omecamtiv mecarbil, a cardiac muscle activator, in patients with heart failure. Additionally, Cytokinetics is developing CK-586, a cardiac myosin inhibitor with a mechanism of action distinct from aficamten for the potential treatment of HFpEF.
For additional information about Cytokinetics, visit www.cytokinetics.com and follow us on X, LinkedIn, Facebook and YouTube.
Forward-Looking Statements
This press release contains forward-looking statements for purposes of the Private Securities Litigation Reform Act of 1995 (the "Act"). Cytokinetics disclaims any intent or obligation to update these forward-looking statements and claims the protection of the Act's Safe Harbor for forward-looking statements. Examples of such statements include, but are not limited to, statements, express or implied, relating to the potential benefits of CK-586 for patients with heart failure with preserved ejection fraction (HFpEF) and our ability to commence a Phase 2 clinical trial of CK-586 in the fourth quarter of 2024, if ever. Such statements are based on management's current expectations, but actual results may differ materially due to various risks and uncertainties, including, but not limited to, potential difficulties or delays in the development, testing, regulatory approvals for trial commencement, progression or product sale or manufacturing, or production of Cytokinetics' drug candidates that could slow or prevent clinical development or product approval; Cytokinetics' drug candidates may have adverse side effects or inadequate therapeutic efficacy; the FDA or foreign regulatory agencies may delay or limit Cytokinetics' ability to conduct clinical trials; Cytokinetics may be unable to obtain or maintain patent or trade secret protection for its intellectual property; standards of care may change, rendering Cytokinetics' drug candidates obsolete; and competitive products or alternative therapies may be developed by others for the treatment of indications Cytokinetics' drug candidates and potential drug candidates may target. For further information regarding these and other risks related to Cytokinetics' business, investors should consult Cytokinetics' filings with the Securities and Exchange Commission.
CYTOKINETICS® and the C-shaped logo are registered trademarks of Cytokinetics in the U.S. and certain other countries.
Contact:
Cytokinetics
Diane Weiser
Senior Vice President, Corporate Affairs
(415) 290-7757
References:
- James et al. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Lancet 2018; 392: 1789–858.
- Bozkurt B, Ahmad T, Alexander KM, Baker WL, Bosak K, Breathett K, Fonarow GC, Heidenreich P, Ho JE, Hsich E, Ibrahim NE, Jones LM, Khan SS, Khazanie P, Koelling T, Krumholz HM, Khush KK, Lee C, Morris AA, Page RL 2nd, Pandey A, Piano MR, Stehlik J, Stevenson LW, Teerlink JR, Vaduganathan M, Ziaeian B; Writing Committee Members. Heart Failure Epidemiology and Outcomes Statistics: A Report of the Heart Failure Society of America. J Card Fail. 2023 Oct;29(10):1412-1451. doi: 10.1016/j.cardfail.2023.07.006. Epub 2023 Sep 26. PMID: 37797885; PMCID: PMC10864030.
- Dunlay SM, Roger VL, Weston SA, Jiang R, Redfield MM. Longitudinal changes in ejection fraction in heart failure patients with preserved and reduced ejection fraction. Circ Heart Fail. 2012 Nov;5(6):720-6. doi: 10.1161/CIRCHEARTFAILURE.111.966366. Epub 2012 Aug 30. PMID: 22936826; PMCID: PMC3661289.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the Management of Heart failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240-e327.
- Jhund PS, MacIntyre K, Simpson CR, et al. Long-Term Trends in First Hospitalization for Heart Failure and Subsequent Survival Between 1986 and 2003. Circulation. 2009;119:515-523.
A graph accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/baf4dfcb-789b-45f8-9ae2-e670fb20f2a8







