Cannabics Pharmaceuticals to Establish a Division for its Antitumor Drug Candidate RCC-33
Cannabics Pharmaceuticals (OTCQB: CNBX) announced the establishment of a dedicated division for its Antitumor Drug Candidate RCC-33, aimed at treating colorectal cancer. This move supports the company’s strategy to enhance clinical validation efforts, including in-vivo experiments and collaborations with medical centers. CEO Eyal Barad emphasized the significance of this initiative, while Medical Director Dr. Erez Scapa highlighted the potential positive impact of RCC-33 on colorectal cancer patients. Cannabics focuses on personalized cannabinoid therapies for cancer.
- Establishment of a dedicated division for RCC-33 indicates a strategic focus on colorectal cancer treatment.
- Plans for clinical validation including in-vivo experiments and collaboration with medical centers.
- Appointment of Dr. Erez Scapa as Medical Director signifies strong leadership for RCC-33's development.
- None.
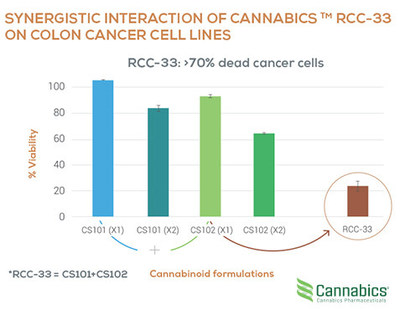
TEL AVIV, Israel and BETHESDA, Maryland, Aug. 20, 2020 /PRNewswire/ -- Cannabics Pharmaceuticals Inc. (OTCQB: CNBX), a leader in personalized cannabinoid medicine focused on cancer and its side effects, announced today that it is establishing a dedicated division for the development of its Antitumor Drug Candidate RCC-33 for the treatment of colorectal cancer.
The announcement comes as the company is gearing up to expand its clinical validation efforts for its RCC-33 formula, and is already preparing a plan for the next steps in its clinical validation path, including in-vivo experiments, collaborations with key medical centers, and the preparation of a product dossier with which the company plans to schedule a Pre IND Meeting with the FDA.
Eyal Barad, Cannabics' CEO commented: "The development of our drug candidate for the treatment of colorectal cancer based on our novel and proprietary formulation RCC-33 has become a central strategic activity for the company. Up until now we have conducted various POC experiments in our in-house drug discovery facility, and at this time, the company has decided to establish a dedicated division to centralize, coordinate and lead all aspects of the development of its drug candidate going forward. Our Scientific Advisory Board Member Dr. Erez Scapa has accepted the position of Medical Director for the new RCC-33 division and assist the company in its forthcoming plans for clinical validation. This is indeed exciting news for the company."
Dr. Erez Scapa commented: "I thank the company for their trust and am looking forward for the opportunity to drive a comprehensive clinical validation plan for the company's drug candidate RCC-33 for colorectal cancer. As a gastroenterologist, I am aware that currently over
About Cannabics Pharmaceuticals
Cannabics Pharmaceuticals Inc. (OTCQB: CNBX) is a U.S public company that is developing a platform that leverages novel drug-screening tools to create cannabinoid-based therapies for cancer that are more precise to a patient's profile. By developing tools to assess effectiveness on a personalized basis, Cannabics is helping to move cannabinoids into the future of cancer therapy. The Company's R&D is based in Israel, where it is licensed by the Ministry of Health to conduct scientific and clinical research on cannabinoid formulations and cancer. For more information, please visit www.cannabics.com. For the latest updates on Cannabics Pharmaceuticals follow the Company on Twitter @Cannabics, Facebook @CannabicsPharmaceuticals, LinkedIn, and on Instagram @Cannabics_Pharmaceuticals.
Disclaimer:
Certain statements contained in this release may constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and other U.S. Federal securities laws. Such statements include but are not limited to statements identified by words such as "believes," "expects," "anticipates," "estimates," "intends," "plans," "targets," "projects" and similar expressions. The statements in this release are based upon the current beliefs and expectations of our Company's management and are subject to significant risks and uncertainties. Actual results may differ from those outlined in the forward-looking statements. Numerous factors could cause or contribute to such differences, including, but not limited to, results of clinical trials and other studies, the challenges inherent in new product development initiatives, the effect of any competitive products, our ability to license and protect our intellectual property, our ability to raise additional capital in the future that is necessary to maintain our business, changes in government policy and regulation, potential litigation by or against us, any governmental review of our products or practices, as well as other risks discussed from time to time in our filings with the Securities and Exchange Commission including, without limitation, our latest 10-Q Report filed July 14th, 2020. We undertake no duty to update any forward-looking statement or any information contained in this press release or other public disclosures at any time. Finally, the investing public is reminded that the only announcements or information about Cannabics Pharmaceuticals Inc., which are condoned by the Company, must emanate from the Company itself and bear our name as its source.
For more information about Cannabics:
Cannabics Pharmaceuticals Inc.
Phone: +1(877)424-2429
info@Cannabics.com
http://www.Cannabics.com
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/cannabics-pharmaceuticals-to-establish-a-division-for-its-antitumor-drug-candidate-rcc-33-301115652.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/cannabics-pharmaceuticals-to-establish-a-division-for-its-antitumor-drug-candidate-rcc-33-301115652.html
SOURCE Cannabics Pharmaceuticals Inc.
FAQ
What is Cannabics Pharmaceuticals announcing in August 2020?
What is the goal of the new division for RCC-33?
Who has been appointed as the Medical Director for the RCC-33 division?
What does Cannabics Pharmaceuticals plan for clinical validation of RCC-33?