BioMarin Announces Positive Phase 3 Gene Therapy Trial Results in Adults with Severe Hemophilia A; Study Met All Primary and Secondary Efficacy Endpoints in One-Year Data Set
BioMarin Pharmaceutical (BMRN) announced positive topline results from its Phase 3 GENEr8-1 study of valoctocogene roxaparvovec, a gene therapy for severe hemophilia A. The trial included 134 participants and found an 84% reduction in Annualized Bleeding Rate (ABR) after one year, with 80% of participants being bleed-free by week five. Factor VIII infusions also decreased by 99%. The FDA granted Breakthrough Therapy Designation, and BioMarin plans to submit data for marketing approval. The study represents a significant step toward a new treatment paradigm for hemophilia A.
- 84% reduction in Annualized Bleeding Rate (ABR) from 4.8 to 0.8 episodes/year (p-value <0.0001).
- 99% decrease in mean Annualized Factor VIII infusions from 135.9 to 2.0 infusions/year (p-value <0.0001).
- 80% of participants became bleed-free starting at week five post-treatment.
- FDA granted Breakthrough Therapy Designation for valoctocogene roxaparvovec.
- Common adverse event: ALT elevation in 86% of participants.
- 16.4% of participants experienced serious adverse events, though all resolved.
Insights
Analyzing...
SAN RAFAEL, Calif., Jan. 10, 2021 /PRNewswire/ -- BioMarin Pharmaceutical Inc. (NASDAQ: BMRN) today announced positive topline results from its ongoing global Phase 3 GENEr8-1 study of valoctocogene roxaparvovec, an investigational gene therapy for the treatment of adults with severe hemophilia A. This is the largest global Phase 3 study to date for any gene therapy in any indication, with 134 participants. All participants in the study received a single dose of valoctocogene roxaparvovec and completed a year or more of follow-up.
Data from the GENEr8-1 Phase 3 study with a mean follow-up of 71.6 weeks showed that in the pre-specified primary analysis for Annualized Bleeding Rate (ABR) a single dose of valoctocogene roxaparvovec significantly reduced ABR by
Valoctocogene roxaparvovec also significantly reduced the mean annualized Factor VIII in the rollover population by
Table 1: Mean/Median Annualized Bleeding Rate (ABR) and FVIII Infusion Rate in Phase 3 GENEr8-1 Study Rollover Population (N=112) from Week 5 Through Week 52 at Nov. 2020 Cut Off
Phase 3 Rollover Population*
On Factor VIII prophylaxis, before
N=112 | Phase 3 Rollover Population*
After valoctocogene roxaparvovec
N=112 | |
Mean (SD) Median (IQR) | Mean (SD) Median (IQR) | |
Annualized Bleeding Rate (bleeding episodes per | 4.8 (6.5) 2.8 (0.0, 7.6) | 0.8 (3.0) 0.0 (0.0, 0.0) |
Annualized FVIII Infusion | 135.9 (52.0) 128.6 (104.1, 159.9) | 2.0 (6.4) 0.0 (0.0, 0.9) |
*See study descriptions for patient population information. |
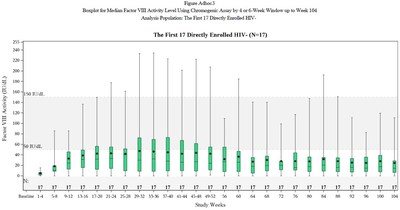
At the end of the first year post-infusion with valoctocogene roxaparvovec, participants in the modified intent-to-treat (mITT) population (N=132) had a mean endogenous Factor VIII expression level of 42.9 (SD 45.5, median 23.9) IU/dL, as measured by the chromogenic substrate (CS) assay, supporting the marked clinical benefits observed with abrogation of bleeding episodes and Factor VIII infusion rate. Factor VIII expression declined at a slower rate compared to the Phase 1/2 study, and remained in a range to provide hemostatic efficacy. In a subset of the mITT population that had been dosed at least two years prior to the data cut date (N=17), Factor VIII expression declined from a mean of 42.2 (SD 50.9, median 23.9) IU/dL at the end of year one to a mean of 24.4 (SD 29.2, median 14.7) IU/dL at the end of year two with continued hemostatic efficacy demonstrated by a mean ABR of 0.9 (median 0.0) bleeding episodes per year.
Table 2: Factor VIII Activity Levels in 6-Month Intervals
Median Factor VIII Activity, IU/dL
| Phase 3 Rollover Population (N=112)
Mean (SD) Median | Phase 3 mITT Subset Population
(N=17*)
Mean (SD) Median | Phase 1/2 6e13 vg/kg Cohort
(N=7)
Mean (SD) Median | Phase 1/2 4e13 vg/kg Cohort
(N=6)
Mean (SD) Median |
Week 26 | 55.1 (57.4) 38.6 | 43.9 (42.1) 33.8 | 71.0 (41.6) 61.2 | 18.0 (8.7) 18.0 |
Week 52 | 43.6 (45.3) 24.2 | 42.2 (50.9) 23.9 | 63.6 (36.5) 60.3 | 21.1 (12.3) 23.8 |
Week 76 | 27.9 (30.6) 15.8 | 53.9 (31.2) 50.2 | 20.6 (15.4) 21.3 | |
Week 104 | 24.4 (29.2) 14.7 | 36.4 (26.3) 26.2 | 12.3 (8.2) 11.6 |
*Includes only HIV-negative subjects dosed 2 or more years prior to Nov 2020 data cut date. One participant was lost to follow-up at 66.1 weeks and was henceforth imputed to have a Factor VIII activity of 0 IU/dL through 104 weeks. |
Please see Figure 1: Box-and-Whiskers Plot.
This is the first statistical evidence demonstrating ABR superiority in a gene therapy trial. These data give us confidence in this groundbreaking alternative to existing therapies and bring us one step closer to a potential new treatment choice to fulfill an unmet medical need for people with hemophilia A," said Steven W. Pipe, MD, Professor of Pediatrics and Pathology, Coagulation Director, Special Coagulation Laboratory Laurence A. Boxer, MD Research Professor of Pediatrics and Communicable Diseases Department of Pathology Michigan Medicine at the University of Michigan and investigator in the Phase 3 study. "This data set adds to the growing body of scientific and clinical data around valoctocogene roxaparvovec gene therapy for hemophilia A and creates the possibility for a new treatment paradigm."
"Over the past seven years, we have conducted rigorous scientific research and clinical programs to address the unmet medical needs of people with severe hemophilia A," said Hank Fuchs, M.D., President of Worldwide Research and Development at BioMarin. "The decades-long aspirations of the hemophilia community are at the forefront of our ongoing commitment to advance this promising investigational gene therapy for the treatment of severe hemophilia A. We are very encouraged by these data and look forward to working with regulatory authorities, treating physicians, and people with hemophilia A to further understand the potential of this gene therapy."
"Although factor replacement therapy has been shown to be a safe and effective treatment modality in hemophilia, it suffers both from incomplete prevention of joint disease and having a high treatment burden with recurring needs for intravenous infusions, which can limit important daily activities out of fear of bleeds," said Guy Young, M.D., Director, Hemostasis and Thrombosis Program at Children's Hospital Los Angeles and Professor of Pediatrics (Clinical Scholar), Keck School of Medicine of University of Southern California. "Novel therapeutic approaches such as gene therapy offer the prospect for both complete prevention of bleeds and subsequent joint damage and eliminating the burden associated with current treatments resulting in an improved quality of life."
Valoctocogene Roxaparvovec Safety
Overall, in the Phase 3 study, valoctocogene roxaparvovec has been well tolerated by the 134 participants who received a single 6e13 vg/kg dose. No participants developed inhibitors to Factor VIII, or thromboembolic events. One participant was lost to follow-up. Infusion-related reactions were effectively mitigated by managing infusion rates.
Alanine aminotransferase (ALT) elevation (115 participants,
Common, steroid-related side effects can occur with temporary use of corticosteroid (or alternative immunosuppressants) to manage ALT elevation. These side effects have generally been grade 1/2 in intensity, manageable and reversible. Isolated grade 3 steroid-related sides effects (e.g., diabetes, hypertension, weight gain, bone fractures) were observed with longer-term higher dose corticosteroid administration. Corticosteroid-related grade 3 SAEs emerged as a safety issue with extended use of corticosteroids which were reversible with only one event of weight gain ongoing.
Overall, in the Phase 1/2 study, the safety profile of valoctocogene roxaparvovec remains consistent with previously reported data with no delayed-onset, treatment-related events. No participants developed inhibitors to Factor VIII, and no participants withdrew from the study. No participants have developed thrombotic events. The most common adverse events associated with valoctocogene roxaparvovec occurred early and included transient infusion-associated reactions and transient, asymptomatic, and mild to moderate rise in the levels of certain proteins and enzymes measured in liver function tests with no long-lasting clinical sequelae.
GENEr8-1 Study Description
The global Phase 3 GENEr8-1 study evaluates superiority of valoctocogene roxaparvovec at the 6e13 vg/kg dose compared to the current standard of care, FVIII prophylactic therapy. All study participants had severe hemophilia A at baseline, defined as less than or equal to 1 IU/dL of Factor VIII activity. The study included 134 total participants, all of whom had a minimum of 12 months of follow-up at the time of the datacut. The first 22 participants were directly enrolled into the Phase 3 study, 17 of whom were HIV-negative and dosed at least 2 years prior to the datacut date (referred to as the subset). The remaining 112 participants (rollover population) completed at least six months in a separate non-interventional study to prospectively assess bleeding episodes, Factor VIII use, and health-related quality of life while receiving Factor VIII prophylaxis prior to rolling over to receive a single infusion of valoctocogene roxaparvovec in the GENEr8-1 study.
Regulatory Status
BioMarin is working with the U.S. Food and Drug Administration (FDA) to align on steps forward to obtain marketing approval for valoctocogene roxaparvovec gene therapy for severe hemophilia A. The FDA recommended that the Company complete the Phase 3 study and submit two-year follow-up safety and efficacy data on all study participants. Additionally, the European Medicines Agency (EMA) requested one-year results from the full Phase 3 study to inform their benefit-risk assessment. To facilitate this submission within the EMA regulatory framework, BioMarin withdrew the MAA and plans to resubmit the MAA with these data to the EMA in the second quarter of 2021 following discussions with the Agency.
The FDA has granted valoctocogene roxaparvovec Breakthrough Therapy Designation. BioMarin's valoctocogene roxaparvovec has received orphan drug designation from the FDA and EMA for the treatment of severe hemophilia A. The Orphan Drug Designation program is intended to advance the evaluation and development of products that demonstrate promise for the diagnosis and/or treatment of rare diseases or conditions.
Call and Webinar to be Held Today, January 10, 2021 at 7:15 PM Eastern Time
Join from a PC, Mac, iPad, iPhone or Android device:
Please click here to join a live Zoom video webinar at 7:15 PM Eastern.
Or join by phone:
Dial (for higher quality, dial a number based on your current location):
US: +1 669 900 6833 or +1 346 248 7799 or +1 253 215 8782 or +1 312 626 6799 or +1 929 205 6099 or +1 301 715 8592
Zoom Webinar ID: 959 1943 2167
International numbers available here.
Phase 1/2 Dose Escalation Study Description
The Phase 1/2 dose escalation study is ongoing and continues to monitor participants long-term. In the study, a total of 15 patients with severe hemophilia A and Factor VIII activity levels less than or equal to 1 IU/dL received a single dose of BMN 270, seven of whom were treated at a dose of 6e13 vg/kg and six of whom were treated at a lower dose of 4e13 vg/kg. The other two participants were treated at lower doses as part of dose escalation in the study and did not achieve therapeutic efficacy.
Robust Clinical Program
BioMarin has multiple clinical studies underway in its comprehensive gene therapy program for the treatment of severe hemophilia A. In addition to the global Phase 3 study GENEr8-1 and the ongoing Phase 1/2 dose escalation study, the Company recently began enrolling participants in a Phase 3b, single arm, open-label study to evaluate the efficacy and safety of valoctocogene roxaparvovec at a dose of 6e13 vg/kg with prophylactic corticosteroids in people with hemophilia A. The Company is running a Phase 1/2 Study with the 6e13kg/vg dose of valoctocogene roxaparvovec in approximately 10 participants with pre-existing AAV5 antibodies, as well as another Phase 1/2 Study with the 6e13 vg/kg dose of valoctocogene roxaparvovec in people with hemophilia A with active or prior FVIII inhibitors.
About Hemophilia A
People living with hemophilia A lack sufficient functioning Factor VIII protein to help their blood clot and are at risk for painful and/or potentially life-threatening bleeds from even modest injuries. Additionally, people with the most severe form of hemophilia A (FVIII levels <
Hemophilia A, also called Factor VIII deficiency or classic hemophilia, is an X-linked genetic disorder caused by missing or defective Factor VIII, a clotting protein. Although it is passed down from parents to children, about 1/3 of cases are caused by a spontaneous mutation, a new mutation that was not inherited. Approximately 1 in 10,000 people have Hemophilia A.
About BioMarin
BioMarin is a global biotechnology company that develops and commercializes innovative therapies for serious and life-threatening rare and ultra-rare genetic diseases. The Company's portfolio consists of six commercialized products and multiple clinical and pre-clinical product candidates. For additional information, please visit www.biomarin.com. Information on BioMarin's website is not incorporated by reference into this press release.
Forward Looking Statements
This press release contains forward-looking statements about the business prospects of BioMarin Pharmaceutical Inc., including without limitation, statements about the development of BioMarin's valoctocogene roxaparvovec program generally, and the Phase 3 results particularly; the impact of valoctocogene roxaparvovec gene therapy for treating patients with severe hemophilia A, the potential for valoctocogene roxaparvovec to reduce or eliminate bleeds, reduce the number of Factor VIII infusions, and the ongoing clinical programs generally. These forward-looking statements are predictions and involve risks and uncertainties such that actual results may differ materially from these statements. These risks and uncertainties include, among others: results and timing of current and planned preclinical studies and clinical trials of valoctocogene roxaparvovec, including final analysis of the above data and additional data from the continuation of these trials; any potential adverse events observed in the continuing monitoring of the patients in the clinical trials; the content and timing of decisions by the FDA, the EMA and other regulatory authorities; the content and timing of decisions by local and central ethics committees regarding the clinical trials; our ability to successfully manufacture the product candidate for the preclinical and clinical trials; and those other risks detailed from time to time under the caption "Risk Factors" and elsewhere in BioMarin's Securities and Exchange Commission (SEC) filings, including BioMarin's Annual and quarterly Reports on Forms 10-K and 10-Q, and future filings and reports by BioMarin. BioMarin undertakes no duty or obligation to update any forward-looking statements contained in this press release as a result of new information, future events or changes in its expectations.
BioMarin® is a registered trademark of BioMarin Pharmaceutical Inc.
Contacts: | |
Investors | Media |
Traci McCarty | Debra Charlesworth |
BioMarin Pharmaceutical Inc. | BioMarin Pharmaceutical Inc. |
(415) 455-7558 | (415) 455-7451 |
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/biomarin-announces-positive-phase-3-gene-therapy-trial-results-in-adults-with-severe-hemophilia-a-study-met-all-primary-and-secondary-efficacy-endpoints-in-one-year-data-set-301204797.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/biomarin-announces-positive-phase-3-gene-therapy-trial-results-in-adults-with-severe-hemophilia-a-study-met-all-primary-and-secondary-efficacy-endpoints-in-one-year-data-set-301204797.html
SOURCE BioMarin Pharmaceutical Inc.